Abstract
In skeletal muscle, extracellular matrix (ECM) remodeling can either support the complete regeneration of injured muscle or facilitate pathologic fibrosis and muscle degeneration. Muscular dystrophy (MD) is a group of genetic disorders that results in a progressive decline in muscle function and is characterized by the abundant deposition of fibrotic tissue. Unlike acute injury, where ECM remodeling is acute and transient, in MD, remodeling persists until fibrosis obstructs the regenerative efforts of diseased muscles. Thus, understanding how ECM is deposited and organized is critical in the context of muscle repair. Connective tissue growth factor (CTGF or CCN2) is a matricellular protein expressed by multiple cell types in response to tissue injury. Although used as a general marker of fibrosis, the cell type–dependent role of CTGF in dystrophic muscle has not been elucidated. To address this question, a conditional Ctgf myofiber and fibroblast-knockout mouse lines were generated and crossed to a dystrophic background. Only myofiber-selective inhibition of CTGF protected δ-sarcoglycan–null (Sgcd−/−) mice from the dystrophic phenotype, and it did so by affecting collagen organization in a way that allowed for improvements in dystrophic muscle regeneration and function. To confirm that muscle-specific CTGF functions to mediate collagen organization, we generated mice with transgenic muscle-specific overexpression of CTGF. Again, genetic modulation of CTGF in muscle was not sufficient to drive fibrosis, but altered collagen content and organization after injury. Our results show that the myofibers are critical mediators of the deleterious effects associated with CTGF in MD and acutely injured skeletal muscle.—Petrosino, J. M., Leask, A., Accornero, F. Genetic manipulation of CCN2/CTGF unveils cell-specific ECM-remodeling effects in injured skeletal muscle.
Keywords: fibrosis, muscular dystrophy, muscle regeneration
After acute injury, transient extracellular matrix (ECM) remodeling promotes normal muscle repair (1). Satellite cells (the endogenous muscle progenitors) rely on ECM plasticity for activation, differentiation, and migration to the injured sites on damaged myofibers (2). In conditions like muscular dystrophy (MD), where genetic defects result in reductions in muscle mass and function, fiber fragility, inflammation, and necrosis drive chronic muscle damage that promotes continual ECM remodeling (3). This process of aberrant ECM remodeling leads to the perpetual stiffening of the ECM and ultimately creates a barrier that impairs skeletal muscle regeneration and repair (4).
In the muscle, resident fibroblasts account for most ECM and fibrotic protein production (5). However, they are not the sole profibrotic cell type in muscle, as the myofibers themselves can promote fibrotic responses and regulate fibroblast activation (6, 7). Regulatory matricellular proteins are expressed by injured myofibers and can exert pleiotropic effects in muscle (8–10). TGF-β has emerged as a primary secreted factor underlying the fibrotic response in MD (11). This proinflammatory molecule, is secreted by injured myofibers, tissue macrophages, and fibroblasts, promotes ECM deposition, fibroblast proliferation, and myofibroblasts differentiation (11–14). Recent work from our group and others has shown that myofiber-specific inhibition of TGF-β signaling protects dystrophic muscles, independent from fibroblast activation (15, 16). This finding places the focus back on myofibers, and highlights their role as overall orchestrators of muscle remodeling in MD.
Connective tissue growth factor (CTGF, or CCN2), is expressed in response to TGF-β signaling and other profibrotic stimuli in muscle (17, 18). It is well known that resident muscle fibroblasts express CTGF (19); however, skeletal muscle myofibers also express CTGF and transiently up-regulate its levels in response to injury (18, 20). In the context of Duchenne MD, reductions in CTGF levels, using heterozygous global loss-of-function mice or neutralizing antibodies against CTGF has been beneficial for disease amelioration (21). These observations suggest that CTGF is a central effector of MD, although the role that CTGF plays throughout disease progression process, within resident muscle cell populations, is unclear.
In the present study, we demonstrated the role of CTGF in dystrophic disease progression that is independent of fibroblast-mediated fibrotic tissue deposition and is mediated by myocyte-specific CTGF. In our study selective inhibition of CTGF in myofibers was sufficient to mitigate MD, independent from the overall extent of fibrosis. Myocyte-derived CTGF acted by modulating ECM relaxation and collagen organization in a way that affected skeletal muscle regeneration.
MATERIALS AND METHODS
Animals
The generation of Ctgf locus of X-over P1 (LoxP)-targeted (fl) mice (Ctgf fl/fl) was described by Liu et al. (22). Ctgf fl/fl mice were crossed with mice expressing the Cre recombinase gene under the control of the skeletal myocyte-specific myogenic differentiation (MyoD) promoter (23) to obtain muscle-restricted deletion of Ctgf [Ctgf skeletal muscle knockout (SkMu-KO)]. To obtain myofibroblast-specific Ctgf deletion, the periostin Mer-Cre-Mer mouse line was adopted (24). Mice that were wild-type for Ctgf, but expressing Cre recombinase were used as controls. Tamoxifen (MilliporeSigma, Burlington, MA, USA) was mixed in sesame oil at 25 mg/ml. Two-week-old mice were given doses of tamoxifen at 0.075 mg/g/d on 7 consecutive days by intraperitoneal injection, after which the mice were maintained on a tamoxifen chow regimen until the terminal time point. Sgcd−/− mice have been described by Hack et al. (25). A modified human skeletal α-actin promoter (26) was used to create transgenic (Tg) mice that overexpressed CTGF. Acute injury was performed by 50 µl intramuscular injection of 1.2% BaCl2 or PBS (control) in the tibialis anterioris. Both males and females were used in the investigations at the ages of 8–24 wk. The use of animals was approved by the Institutional Animal Care and Use Committee at The Ohio State University. No human subjects or materials were used.
ELISA, hydroxyproline assay, and creatine kinase measurement
ELISA assays were performed on muscle homogenates with an ELISA kit for TGF-β1 purchased from R&D Systems (SMB100B; Minneapolis, MN, USA). Hydroxyproline content was determined, as described by Accornero et al. (27). Creatine kinase (CK) levels were obtained from serum as previously described (28).
Fiber and fibroblast isolation
Muscle fibers were isolated by incubating quadriceps muscle, with intact tendons, in 0.2% collagenase (type I, C-0130; MilliporeSigma) for 2 h at 37°C with gentle agitation (100 rpm). After collagenase treatment, muscles were transferred to DMEM (11995065; Thermo Fisher Scientific, Waltham, MA, USA) containing 10% horse serum to deactivate the collagenase solution. To release individual fibers, digested muscles were then incubated with heat-inactivated horse serum, and myofiber release was facilitated by gently mixing the solution with a Pasteur pipette. Myofibers were then centrifuged at 1000 g. Pelleted myofibers were washed 2 times with PBS during low-speed centrifugation, and the final pellet was processed for RNA extraction.
For fibroblast isolation, the suspension obtained after muscle digestion, which contains interstitial cells, was plated in DMEM enriched with 10% bovine serum to obtain adherence and growth of resident muscle fibroblasts.
Histologic analysis and immunohistochemistry
Muscles were embedded in paraffin, and 5-μm histologic sections were cut at the center of the muscle and stained with hematoxylin and eosin (H&E), Picrosirius red (PSR), or Masson’s trichrome. Quantifications of myofiber cross-sectional area and fibrosis were obtained with ImageJ software [National Institutes of Health (NIH), Bethesda, MD, USA]. For fibrosis quantification with trichrome staining, the area of blue-stained fibrosis was quantified relative to the total muscle area (29). We used PSR staining, according to the manufacturer’s recommendations (24901-500; Polysciences, Warrington, PA, USA), to visualize the collagen network under polarized light, and quantifications were obtained with ImageJ software. For analysis, polarized images were analyzed and the amounts of densely (red) and loosely (green) packed collagen were quantified in the muscle cross sections relative to the total collagen area, by the ImageJ red-green-blue quantification feature. Immunohistochemistry for CTGF was performed with antibodies from Santa Cruz Biotechnology (sc-14939; Dallas, TX, USA). Wheat germ agglutinin (Vector Laboratories, Burlingame, CA, USA) was used to outline cell borders.
mRNA expression analysis
RNA was extracted from muscles with the RNeasy Kit according to the manufacturer’s instructions (Qiagen, Germantown, MD, USA). Samples were reverse transcribed for standard real-time quantitative PCR (qPCR) assays. Reverse transcription was performed with the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Selected gene differences were analyzed by real-time qPCR with Sso-Advanced Universal Sybr Green Supermix (Bio-Rad, Hercules, CA, USA). Quantified mRNA expression was normalized to the housekeeping gene Rpl7 and expressed relative to controls. Quantifications were performed with the 2ΔΔCt method. Specifically, values were first normalized to the housekeeping gene Rpl7. ΔCt was then calculated by subtracting the target gene mean Ct (mean of technical triplicates) from the mean Rpl7 Ct (mean of technical triplicates) to generate ΔCt values. The mean ΔCt values for all the control samples were then use to generate ΔΔCt values by taking each sample ΔCt and subtracting it from the sum of the mean control ΔCt values. Fold change was then generated from the ΔΔCt values.
Lysyl oxydase activity analysis
Fluorometric lysyl oxydase (LOX) activity assays were performed with quadriceps muscle homogenates with a kit purchased from BioVision (K978-100; Milpitas, CA, USA) according to the company’s instructions. Relative fluorescence units were collected every 30 s for 30 min (excitement/emission 500/536 µm) in kinetics mode. Specific detected activity was then calculated with the following equation (Eq. 1):
where ΔM is the linear change in oxidized probe concentration during Δt (pmol); Δt = t2 − t1 (min) is the difference in time between the first and last time points; and V is the sample protein content added to the well (µg).
Forced treadmill running
Mice were placed in individual lanes of an electrically driven 4-lane treadmill (Omni-Pacer LC4/M; Columbus Instruments International, Columbus, OH, USA) at the speed of 6 M/min for 3 min. The treadmill measured 48 cm wide × 51 cm in length, with a conveyor belt measuring 8 cm wide by 30 cm in length. A training regimen was first instituted for 10 min to familiarize the mice with the environment and the shock grids (adjustable from 0 to 2.0 mA). The speed was increased in increments of 2 M/min every 3 min to a maximum speed of 22 M/min. Exhaustion was assessed as >5 consecutive seconds on the shock grid without attempting to re-engage the treadmill, as described by Petrosino et al. (30). Time spent on the treadmill before exhaustion or time to complete the protocol was recorded. The regimen was performed with the treadmill in downhill mode.
Statistics
All results are means ± sem. Statistical analysis was performed with a Mann-Whitney U test, or 2-tailed Student’s t test for normally distributed data. Values of P < 0.05 were considered significant. For groups of ≥3, univariate ANOVAs with subsequent Tukey honest significant difference multiple comparisons were performed, with significance set at α = 0.05.
RESULTS
Generation of myofibroblast- and myocyte-specific CTGF-KO mice
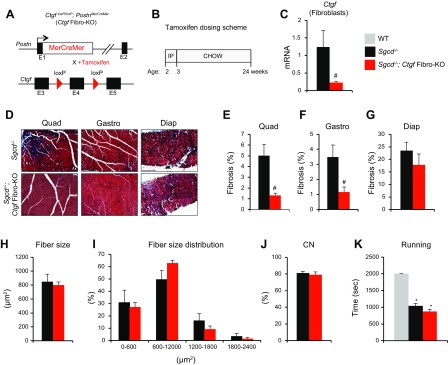
CTGF is produced in skeletal muscle, and its expression is elevated in MD, where it is known to have a negative impact on muscle health and to promote fibrosis (17, 21). Despite the association between CTGF and dystrophic disease progression, the cell-specific contribution of CTGF to dystrophy pathophysiology has not been studied. Fibroblasts are crucial mediators of organ fibrosis (5). When activated, they transformed into collagen-producing myofibroblasts, which are characterized by expression of the marker periostin (24). To dissect the specific role of CTGF in skeletal muscle fibrosis, we first used a tamoxifen-inducible Cre recombinase–expressing mouse line under the control of the periostin promoter and crossed it with a conditional Ctgf allele (Ctgf Fibro-KO) (Fig. 1A). Myofibroblast-specific CTGF deletion mice were then crossed with the δ-sarcoglycan null (Sgcd−/−) mouse model of limb–girdle MD type 2F, a model that produces fulminant dystrophic disease a few weeks after birth (25, 31). Tamoxifen was administered for 24 wk until the completion of the experiment (Fig. 1B). Tamoxifen treatment successfully deleted Ctgf from isolated fibroblasts (Fig. 1C). Histologic analysis of fibrosis by Masson’s trichrome staining revealed a reduction in fibrotic deposition in the quadriceps and gastrocnemius; however, we did not observe differences in the amount of fibrosis in the diaphragm (Fig. 1D–G). To further assess whether deletion of CTGF in fibroblasts affects the dystrophic phenotype, we analyzed myofiber size and central nucleation and found no differences in these parameters of muscle pathophysiology (Fig. 1H–J). In addition, functional testing by treadmill running showed that myofibroblast-specific deletion of CTGF had no effect on dystrophic running performance (Fig. 1K). Using this genetic approach, we were able to determine that the fibrotic response driven by myofibroblast CTGF production does not affect the overall dystrophic phenotype. Considering that CTGF can also be expressed by myocytes, these early findings led us to hypothesize that muscle-derived CTGF drives the deleterious MD pathology.
Figure 1 .
Generation of myofibroblast-specific CTGF KO mice. A) Strategy for the generation of an inducible myofibroblast-specific Ctgf-deletion mouse line (Ctgf Fibro-KO). B) Tamoxifen dosing strategy. Chow, normal diet; IP, intraperitoneal. C) Ctgf expression relative to housekeeping gene Rpl7 analysis by real-time qPCR in isolated fibroblasts from Sgcd−/− quadriceps or Sgcd−/− Ctgf Fibro-KO (n = 4 each). D) Representative Masson’s trichrome–stained histologic sections of the indicated muscle groups and genotypes. Scale bars, 200 μm. E–G) Quantification of trichrome fibrotic staining (n = 7 Sgcd−/− and n = 4 Sgcd−/−Ctgf Fibro-KO). H, I) Mean fiber size (H) and fiber size distribution (I) analysis of myofiber cross-sectional areas quantified from quadriceps muscles of the indicated genotypes (n = 4 each). J) Quantification of the percentage of myofibers with centrally located nuclei from H&E-stained histologic sections from quadriceps muscles of the indicated genotypes (n = 5 each). CN, central nucleation. K) Time to fatigue in seconds during forced downhill treadmill running (n = 4 WT, n = 9 Sgcd−/−, and n = 8 Sgcd−/−Ctgf Fibro-KO). Diap, diaphragm; Gastro, gastrocnemius; Quad, quadriceps;. *P < 0.05 vs. nondystrophic WT controls, #P < 0.05 vs. Sgcd−/− mice.
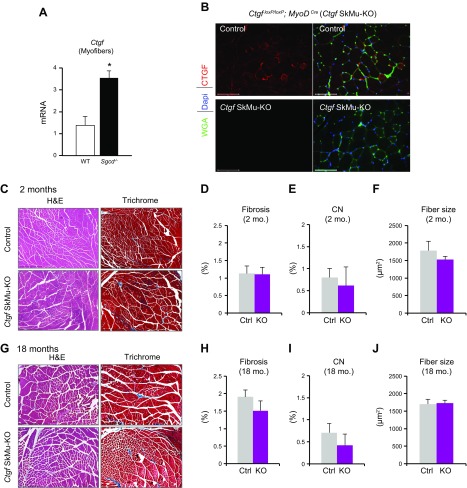
We found that myofibers are a significant source of CTGF and that Ctgf expression is increased in dystrophic myofibers isolated from the Sgcd−/− mouse model (Fig. 2A). To define the contribution of myofiber-derived CTGF for muscle homeostasis and disease, we generated skeletal myocyte-specific Ctgf gene-targeted mice in which deletion of CTGF was obtained by crossing Ctgf loxP/loxP mice with mice expressing Cre recombinase under the control of the myocyte-specific MyoD promoter. Ctgf loxP/loxP mice carrying the MyoD Cre transgene (SkMu-KO) showed successful deletion of CTGF in myofibers (Ctgf SkMu-KO) (Fig. 2B). H&E and Masson’s trichrome–stained histologic sections showed no obvious phenotype in the muscles of Ctgf SkMu-KO mice compared with those of control littermates at 2 mo of age (Fig. 2C). No differences in fibrosis, central nucleation, or fiber size were observed (Fig. 2D–F). To test whether aging would highlight a phenotype in Ctgf SkMu-KO, we then analyzed muscles from 18-mo-old mice. Even with aging, we did not observe abnormalities by deleting CTGF within myocytes by histopathologic analysis of fibrosis, central nucleation or fiber size (Fig. 2G–J). Thus, deletion of CTGF from skeletal muscle fibers does not perturb muscle homeostasis.
Figure 2 .
Generation of skeletal myocyte-specific CTGF-KO mice. A) CTGF expression analysis by real-time qPCR in isolated WT or dystrophic Sgcd−/− myofibers. Expression was normalized to theRpl7 housekeeping gene (n = 4 each). B) Immunohistochemistry of quadriceps cross sections with CTGF (red), wheat germ agglutinin to outline cell borders (green), and DAPI staining denoting nuclei (blue). Scale bars, 100 μm. C) H&E and Masson’s trichrome–stained representative histologic sections from the quadriceps of 2-mo-old mice. Scale bars, 500 μm. D–F) Quantification of trichrome fibrotic staining (D), central nucleation (E), and mean fiber size (F) from the quadriceps of 2-mo-old mice. G) H&E and Masson’s trichrome–stained representative histologic sections from the quadriceps of 18-mo-old mice. Scale bars, 500 μm. H–J) Quantification of trichrome fibrotic staining (H), central nucleation (I), and mean fiber size (J) from the quadriceps of 18-mo-old mice (n = 4 each quantification at 2 mo; n = 5 each quantification at 18 mo). *P < 0.05 vs. WT.
Myofiber-specific deletion of CTGF mitigates MD pathophysiology
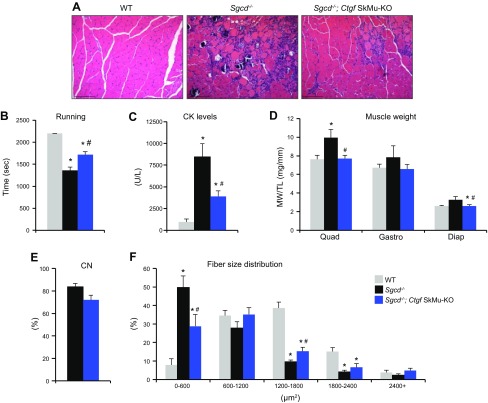
Myocyte deletion of CTGF did not produce a noticeable phenotype; however, in muscle, CTGF expression was low at baseline and increased after injury. We proceeded to test the contribution of myocyte CTGF in the setting of MD, where CTGF levels are elevated. To do this, we crossed the Ctgf SkMu-KO onto the Sgcd−/− genetic background. Deletion of CTGF in myofibers ameliorated Sgcd−/− mouse histopathology (Fig. 3A). To assess whether the observed improvement in histopathology that was conferred by the muscle-specific CTGF deletion correlated with functional benefits, we subjected mice to forced treadmill running. Sgcd−/− mice with CTGF deletion in myofibers showed an improvement of their ability to run on a treadmill at 2 mo of age, whereas Sgcd−/− mice alone were severely compromised (Fig. 3B). Sgcd−/− mice with Ctgf SkMu-KO also showed a significant reduction in total serum CK, suggesting less ongoing myofiber necrosis (Fig. 3C). In addition, we observed a normalization of the pseudohypertrophy typically observed in young dystrophic muscles (Fig. 3D). To assess myogenic repair, we then analyzed the molecular profile and morphology of regenerating myofibers. Immature myofibers attempting to regenerate are characterized by the re-expression of myogenin (Myog) and embryonic myosin heavy chain 3, and the size of these fibers determines their ability to complete the regeneration process. We found that expression of both Myog and myosin heavy chain 3 was significantly reduced in dystrophic muscles lacking myocyte-derived CTGF (Supplemental Fig. 2). However, the overall number of centrally nucleated dystrophic myofibers was not modified by the loss of muscle CTGF (Fig. 3E). That central nucleation was not significantly affected by myocyte-derived CTGF indicated that an equal number of injured fibers participated in the regenerative repair. Additional analysis of fiber size distribution revealed a normalization of myofiber area in dystrophic muscle without CTGF to that of WT animals (Fig. 3F). More specifically, there was a reduction in the number of small myofibers (immature) and an overall shift in fiber size distribution toward the control uninjured WT condition (Fig. 3F). These results suggest that inhibiting CTGF specifically in myofibers increases the extent of successful, complete regeneration and myofiber maturation in dystrophic muscle. These combined observations further suggest that myofiber-derived CTGF plays a primary role in dystrophic disease pathophysiology.
Figure 3 .
Myofiber-specific deletion of CTGF mitigates MD pathology. A) Representative H&E-stained histologic sections from the quadriceps of 2-mo-old mice. Scale bars, 200 μm. B) Time to fatigue in seconds with forced downhill treadmill running in 2-mo-old mice (n = 4 WT, n = 5 Sgcd−/−, and n = 7 Sgcd−/−Ctgf SkMu-KO). C) Quantification of CK levels in the blood of 2-mo-old mice (n = 4 WT, n = 5 Sgcd−/−, and n = 7 Sgcd−/−Ctgf SkMu-KO). D) Muscle weights (MW) normalized to tibia length (TL) from the indicated muscle groups of mice at 2 mo of age (n = 4 WT, n = 5 Sgcd−/−, and n = 7 Sgcd−/−Ctgf SkMu-KO). Diap, diaphragm; Gastro, gastrocnemius; Quad, quadriceps. E) Quantification of the percentage of myofibers with centrally located nuclei from H&E-stained histologic sections from quadriceps muscles of the indicated genotypes at 2 mo of age (n = 5 each). CN, central nucleation. F) Fiber size distribution analysis of myofiber cross-sectional areas quantified from quadriceps muscles of the indicated genotypes at 2 mo of age (n = 3 WT, n = 5 Sgcd−/−, and n = 5 Sgcd−/−Ctgf SkMu-KO). *P < 0.05 vs. WT, #P < 0.05 vs. Sgcd−/− mice.
Myofiber-derived CTGF functions in the repair process by regulating ECM organization
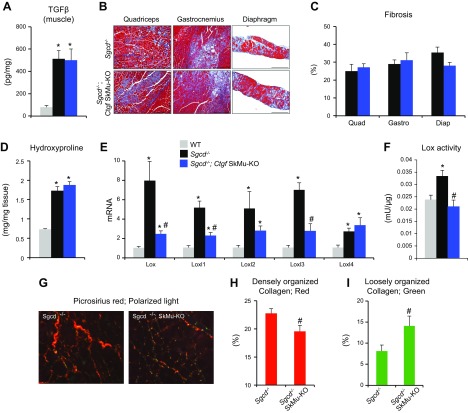
We next tested whether CTGF derived from myofibers affects dystrophic disease progression by regulating the deposition of fibrotic tissue, as well as the dependency of TGF-β bioavailability as an upstream regulator in this process. As expected, TGF-β was strongly induced in dystrophic muscles, but the deletion of CTGF from myofibers did not alter TGF-β levels (Fig. 4A). Despite the protective features associated with inhibition of CTGF within the myofibers, quantification of total muscle fibrosis by trichrome staining analysis revealed no significant changes in the fibrosis content of Sgcd−/− mice, with or without myofiber CTGF (Fig. 4B, C). We next measured the expression of the main collagen types responsible for muscle fibrosis: collagen I and III. We observed an expected induction of both collagen I and III in the dystrophic models, and, as expected, based on the observed pathology, found only a trend toward a reduction in the collagens in the dystrophic mice with CTGF deletion in myocytes (Supplemental Fig. 1). These observations suggest that the primary role of CTGF in the myofibers is independent of the well-established profibrotic effect.
Figure 4 .
Myofiber-derived CTGF does not contribute to muscle fibrosis. A) ELISA for TGF-β levels in muscles from 2-mo-old (n = 3 WT, n = 4 Sgcd−/−, and n = 4 Sgcd−/−Ctgf SkMu-KO). B) Representative Masson’s trichrome–stained histologic sections of the indicated muscle groups and genotypes at 2 months. Scale bars, 200 μm. C) Quantification of trichrome fibrotic staining. Diap, diaphragm; Gastro, gastrocnemius; Quad, quadriceps. (n = 4 each). D) Measurement of hydroxyproline content from quadriceps muscles of the indicated genotypes at 2 months (n = 3 WT, n = 4 Sgcd−/−, and n = 5 Sgcd−/−Ctgf SkMu-KO). E) Expression analysis for Lox and Loxl1, -2, -3, and -4 normalized to the Rpl7 housekeeping gene from the quadriceps muscles of the indicated genotypes at 2 months (n = 3 WT, n = 4 Sgcd−/−, and n = 5 Sgcd−/−Ctgf SkMu-KO). F) Quantification of Lox activity in quadriceps muscle protein extracts from 2-mo-old mice (n = 3 WT, n = 4 Sgcd−/−, and n = 3 Sgcd−/−Ctgf SkMu-KO). G) Representative PSR-stained images visualized with polarized light microscopy. Original magnification, ×20. H, I) Quantification of collagen bundling from picrosirius images under polarized light showing densely (red; H) and loosely (green; I) packed collagen (n = 7 each). *P < 0.05 vs. WT, #P < 0.05 vs. Sgcd−/− mice.
To examine the mechanism whereby muscle-specific inhibition of CTGF is protective, we looked more closely at ECM composition, which is critical for muscle repair and integrity. We first conducted an unbiased examination of total collagen deposition and then independently examined the collagen network. In agreement with our trichrome analysis shown in Fig. 4C, the total collagen content, although induced in dystrophy, was unaffected by ablation of myocyte-derived CTGF (hydroxyproline content assay, Fig. 4D). The expression of the collagen cross-linkers Lox and Loxl1 and Loxl3, which were increased in dystrophy, were down-regulated in dystrophic myocytes lacking CTGF (Fig. 4E). Decreased levels of Lox genes correlated with reduction in overall Lox activity in muscles from dystrophic mice crossed with Ctgf SkMu-KO (Fig. 4F). This result suggests that overall dystrophic fibrotic tissue deposition is unaffected by myocyte CTGF, but that muscle-specific CTGF may modulate the dystrophic phenotype by affecting ECM composition and collagen stiffness. To further investigate this notation, we then analyzed the collagen network of dystrophic muscle, with or without myofiber-derived CTGF, using polarized light microscopy and PSR staining (32). When PSR-stained muscle sections from Sgcd−/− mice or Sgcd−/− Ctgf SkMu-KO animals were analyzed under polarized light, we observed a decreased red densely packed collagen in dystrophic muscle lacking CTGF and increased green loosely packed collagen (Fig. 4G–I). Thus, the reduction in tight collagen bundling and its association with improved functionality indicate that myofiber-derived CTGF controls the organization of collagen fibrils, which in turn regulates the antiregenerative phenotype observed in MD.
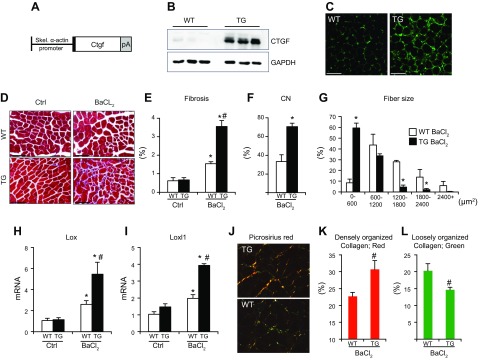
To confirm that CTGF truly regulates the organization of collagen fibrils, we generated a muscle-specific CTGF-overexpressing mouse model, driven by the muscle-specific skeletal α-actin promoter (Fig. 5A), and investigated how overexpression of muscle CTGF affects fibrosis and ECM organization. Up-regulation of CTGF levels was confirmed by Western blot analysis and immunostaining (Fig. 5B, C). Increased amounts of CTGF did not drive fibrosis at baseline (Fig. 5D; right panels). To determine whether the effects of CTGF were specific to conditions of regeneration, we injected tibialis anterioris with BaCl2, a chemical commonly used to injure skeletal muscles. After injury, we found that CTGF TG mice had a greater fibrotic response, as assessed by histologic analysis of Masson’s trichrome–stained sections and qPCR analysis of collagen I and III expression (Fig. 5E and Supplemental Fig. 3A, B). To assess whether the observed aberrant fibrotic response results from defective regeneration after injury, we subsequently analyzed central nucleation, fiber size distribution, and expression of regeneration markers. We found that CTGF overexpression interfered with muscle repair in response to BaCl2, indicated by the substantially greater number of centrally nucleated myofibers in CTGF TG muscles 14 d after injury (Fig. 5F). In addition, overexpression of CTGF led to sustained expression of Myog after administration of BaCl2, indicating aberrant regenerative response and inability to resolve the injury (Supplemental Fig. 3C). The defective regeneration in CTGF Tg mice also led to a profound shift in fiber size distribution after BaCl2, suggesting impaired reparative muscle growth (Fig. 5G). Considering that myofiber-derived CTGF KO resulted in down-regulation of the collagen cross-linking enzymes, we reasoned that ECM organization could be responsible for the defective regeneration capacity of CTGF-overexpressing muscles. To confirm this theory, we looked at the expression of Lox and Loxl1 and found that overexpression of CTGF up-regulated these enzymes after BaCl2 injury (Fig. 5H, I). In addition, analysis of the collagen network with polarized light microscopy and PSR staining revealed that overexpression of muscle CTGF promoted an increase in the amount of densely packed collagen in injured muscles (Fig. 5J–L). These data confirm that CTGF levels modulate the response of skeletal muscle to injury by modifying ECM composition and organization.
Figure 5 .
Generation and analysis of CTGF-overexpressing Tg mice. A) The transgene strategy (pA, poly A). B) Western blot analysis of CTGF protein expression and GAPDH loading control. C) Immunostaining for CTGF (green) in cross sections of the quadriceps. Scale bars, 100 μm. D) Representative Masson’s trichrome–stained histologic sections at 2 months. Ctrl, PBS control. Scale bars, 100 μm. E) Quantification of trichrome fibrotic staining. F) Quantification of the percentage of myofibers with centrally located nuclei from H&E-stained histologic sections from tibialis anterioris muscles CN, central nucleation. G) Fiber size distribution analysis of myofiber cross-sectional areas quantified from tibialis anterioris muscles H, I) Real-time qPCR analysis for expression of Lox (H) and Loxl1 (I) normalized to Rpl7. J) Representative PSR-stained images visualized with polarized light microscopy. Original magnification, ×40. K, L) Quantification of collagen bundling from PSR-stained images under polarized light showing densely (red; K) and loosely (green; L) packed collagen (n = 4 each). *P < 0.05 vs. uninjured controls, #P < 0.05 vs. WT mice same treatment.
DISCUSSION
Fibrosis is a process that is largely controlled by resident tissue fibroblast activation and their subsequent expression of collagens and other connective tissue components (5, 33). For years, elevated levels of CTGF (or CCN2) have been positively associated with fibrosis, and the role of CTGF in modulating fibroblast activation and their profibrotic phenotype has been well characterized (34). As a result, this secretory protein has become a widely known marker of fibrosis; however, some reports have shown that CTGF is not just a profibrotic marker, but rather, may have a causative role in this process (21, 35). As the result of its being a secreted factor, not much attention has been placed on understanding the role of CTGF in different cells of origin. However, our previous work on cardiac fibrosis led us to find that this protein is highly expressed by cardiomyocytes after injury, but that its expression in these cells is unimportant for overall collagen deposition (36). These results have been confirmed by several independent groups and raised the intriguing possibility that CTGF is involved in different functions based on its source (37, 38).
In skeletal muscle, the injury–repair–regeneration cascade acutely promotes transient collagen deposition (39–42). When skeletal muscle is exposed to chronic injuries, as in conditions of MD, these deposits accumulate, impair the regenerative process, and ultimately result in the replacement of muscle by fibrotic tissue (43–45). CTGF has been extensively studied as a profibrotic factor and as a marker of chronic diseases, such as MD (46). To date, only global genetic and therapeutic approaches have been applied to modulate CTGF levels and function, ultimately leaving the question as to what the cell-specific biology of this matricellular protein is. In respect to the role of this protein in muscle biology, studies adopting global heterozygous mice expressing half of the endogenous amount of CTGF showed that when crossed to the MDX mouse model of Duchenne MD, reduced levels of CTGF ameliorated dystrophic disease progression by limiting the extent of fibrosis (21). Similarly, global targeting of CTGF using neutralizing antibodies has been beneficial in limiting fibrosis in the MDX mouse model (21). The above-mentioned studies, together with the several reports indicating that CTGF is strongly elevated in patients and mice with MD, highlight the importance of understanding the biology of CTGF in skeletal muscle (47, 48).
Our work and the work of others have found that, in skeletal muscle, CTGF is expressed by fibroblasts and myofibers (19, 20). Deletion of CTGF specifically in fibroblasts decreased hindlimb fibrosis in the context of MD, suggesting that targeting fibroblast-derived CTGF in muscle would be protective. However, in our model, this was not sufficient to functionally rescue dystrophic running impairment, suggesting a more complex role for this matricellular protein in skeletal muscle. Myofibers from our Sgcd−/− mouse model of limb-girdle MD showed up-regulated CTGF expression. This finding is in line with our previous observation in the heart that myocytes represent an important source of CTGF during injuries. Similarly, our cell-type–specific genetic approach used in this study revealed that deletion of CTGF in myofibers, although protective, does not act by modulating overall collagen content. This finding agrees with unchanged TGF-β levels and with the fact that our genetic manipulation was myocyte specific and did not target fibroblasts. However, the results did not per se exclude the possibility that myofibers can use CTGF to affect the composition of the ECM beyond collagen content.
The skeletal muscle ECM is a complex network of structural and signaling components that play important regulatory function in controlling muscle physiology in homeostasis and during injury (1, 49). In addition to providing mechanical support to the sarcolemma, the ECM also constitutes the microenvironment of satellite cells, the natural skeletal muscle resident stem cell population (50). Satellite cells are responsible for the robust regenerative capacity of skeletal muscles, a hallmark that distinguishes skeletal and cardiac muscle, the latter of which is largely nonregenerative (51). Although proregenerative, in conditions of chronic injury such as in MD, satellite cells lose their ability to self-renew, thus reducing their potential to undergo myogenic differentiation and repair injured myofibers (4). Changes in the surrounding microenvironment can have a profound impact on satellite cell function. ECM abnormalities and stiffening can create a barrier that impairs satellite cell migration to injury sites (42, 52). These types of malignant changes in the extracellular meshwork architecture are observed in MD and are regulated by cross-linking enzymes such as LOXs (53). LOXs function as ECM cross-linking enzymes that mediate collagen density and organization (54). In muscle, LOX, like CTGF, is secreted by myofibers and plays a crucial part in myogenesis (55). In dystrophic muscle from mice, dogs, and humans, levels of LOX are increased and coincide with increased collagen cross-linking (43, 53). Similar to CTGF, LOX was recently shown to be a core gene involved in MD pathogenesis (53).
We found that myofiber-derived CTGF modulates collagen fibril density in muscle. In a model of acute regeneration, in which transient ECM remodeling is necessary for complete regeneration, we found that overexpression of CTGF affected the skeletal muscle injury-response by favoring fibrosis and up-regulating the LOX genes in response injury. LOX functions to promote more heavily cross-linked collagen fibrils and subsequently promotes more densely packed collagen networks (56). Consistent with the role of LOX in collagen cross-linking, we also observed phenotypic changes in the Tg mice that was consistent with an increase in densely packed collagen fibrils. Aberrant ECM organization led to impaired muscle regeneration in CTGF overexpressing mice, which showed defective muscle differentiation and reparative growth. It is likely that the increase in fibrotic deposition observed in this postinjury model reflects the inhibitory effect of CTGF for muscle repair. The role of CTGF as a modulator of muscle ECM was further demonstrated in the dystrophic mice. In this chronic-injury model, where CTGF is already elevated, dystrophic myocyte–specific deletion of CTGF resulted in down-regulation of the LOX enzymes. Although somewhat debated, probably because of the means of quantification, the presence of densely packed collagen networks has been shown in MD and appear to be more indicative of ECM stiffness than collagen content alone (43, 57). If CTGF is a mediator of LOX expression, then the functional consequence would be an effect on the collagen networks in dystrophic muscles. We observed that dystrophic CTGF-null myocytes had a shift from densely packed collagen networks to a more loosely packed collagen architecture, which ultimately resulted in improvements in the function, size, and maturity of regenerating myofibers. Our results suggest that CTGF functions as a malignant orchestrator of ECM architecture in dystrophic myofibers and that CTGF-mediated remodeling of the ECM compromises muscle regeneration.
In this study, myofibers were important mediators of the deleterious effects of CTGF in muscle. Our results demonstrate that targeting CTGF in committed myogenic cells counteracted the stiffening of the collagen network that is characteristic of dystrophic muscle, without affecting overall collagen content. Therefore, global CTGF inhibition may be a good strategy for reducing collagen deposition and the extent of fibrosis, and targeted inhibition of CTGF at the level of the myofibers may provide enhanced benefits for modulating the ECM architecture in conditions where collagen deposition is needed for proper repair and function.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by the U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (Grants R00HL121284 and R01HL136951 to F.A.), NIH National Institute of General Medical Sciences (Grant T32GM068412), and NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grant F31AR073638 to J.M.P.). The authors declare no conflicts of interest.
Glossary
- CK
creatine kinase
- CTGF
connective tissue growth factor
- ECM
extracellular matrix
- H&E
hematoxylin and eosin
- LOX
lysyl oxidase
- LoxP
locus of X-over P1
- MD
muscular dystrophy
- MyoD
myogenic differentiation
- Myog
myogenin
- PSR
Picrosirius red
- qPCR
quantitative PCR
- Sgcd
δ-sarcoglycan
- SkMu-KO
skeletal muscle knockout
- Tg
transgenic
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J. M. Petrosino performed the research and analyzed the data; A. Leask contributed new reagents; and F. Accornero designed research and wrote the manuscript.
REFERENCES
- 1.Gillies, A. R., Lieber, R. L. (2011) Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 44, 318–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calve, S., Simon, H. G. (2012) Biochemical and mechanical environment cooperatively regulate skeletal muscle regeneration. FASEB J. 26, 2538–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallace, G. Q., McNally, E. M. (2009) Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu. Rev. Physiol. 71, 37–57 [DOI] [PubMed] [Google Scholar]
- 4.Cohn, R. D., van Erp, C., Habashi, J. P., Soleimani, A. A., Klein, E. C., Lisi, M. T., Gamradt, M., ap Rhys, C. M., Holm, T. M., Loeys, B. L., Ramirez, F., Judge, D. P., Ward, C. W., Dietz, H. C. (2007) Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states [published correction appears in Nat. Med. (2007) 13, 511]. Nat. Med. 13, 204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman, M. A., Meza, R., Lieber, R. L. (2016) Skeletal muscle fibroblasts in health and disease. Differentiation 92, 108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fry, C. S., Kirby, T. J., Kosmac, K., McCarthy, J. J., Peterson, C. A. (2017) Myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem Cell 20, 56–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamar, K. M., Bogdanovich, S., Gardner, B. B., Gao, Q. Q., Miller, T., Earley, J. U., Hadhazy, M., Vo, A. H., Wren, L., Molkentin, J. D., McNally, E. M. (2016) Overexpression of latent TGFβ binding protein 4 in muscle ameliorates muscular dystrophy through myostatin and TGFβ. PLoS Genet. 12, e1006019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merly, F., Lescaudron, L., Rouaud, T., Crossin, F., Gardahaut, M. F. (1999) Macrophages enhance muscle satellite cell proliferation and delay their differentiation. Muscle Nerve 22, 724–732 [DOI] [PubMed] [Google Scholar]
- 9.Lescaudron, L., Peltékian, E., Fontaine-Pérus, J., Paulin, D., Zampieri, M., Garcia, L., Parrish, E. (1999) Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscul. Disord. 9, 72–80 [DOI] [PubMed] [Google Scholar]
- 10.Brunelli, S., Rovere-Querini, P. (2008) The immune system and the repair of skeletal muscle. Pharmacol. Res. 58, 117–21 [DOI] [PubMed] [Google Scholar]
- 11.Bernasconi, P., Torchiana, E., Confalonieri, P., Brugnoni, R., Barresi, R., Mora, M., Cornelio, F., Morandi, L., Mantegazza, R. (1995) Expression of transforming growth factor-beta 1 in dystrophic patient muscles correlates with fibrosis: pathogenetic role of a fibrogenic cytokine. J. Clin. Invest. 96, 1137–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iannaccone, S., Quattrini, A., Smirne, S., Sessa, M., de Rino, F., Ferini-Strambi, L., Nemni, R. (1995) Connective tissue proliferation and growth factors in animal models of Duchenne muscular dystrophy. J. Neurol. Sci. 128, 36–44 [DOI] [PubMed] [Google Scholar]
- 13.Murakami, N., McLennan, I. S., Nonaka, I., Koishi, K., Baker, C., Hammond-Tooke, G. (1999) Transforming growth factor-beta2 is elevated in skeletal muscle disorders. Muscle Nerve 22, 889–898 [DOI] [PubMed] [Google Scholar]
- 14.Sun, G., Haginoya, K., Dai, H., Chiba, Y., Uematsu, M., Hino-Fukuyo, N., Onuma, A., Iinuma, K., Tsuchiya, S. (2009) Intramuscular renin-angiotensin system is activated in human muscular dystrophy. J. Neurol. Sci. 280, 40–48 [DOI] [PubMed] [Google Scholar]
- 15.Accornero, F., Kanisicak, O., Tjondrokoesoemo, A., Attia, A. C., McNally, E. M., Molkentin, J. D. (2014) Myofiber-specific inhibition of TGFβ signaling protects skeletal muscle from injury and dystrophic disease in mice. Hum. Mol. Genet. 23, 6903–6915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein, J. A., Bogdanovich, S., Beiriger, A., Wren, L. M., Rossi, A. E., Gao, Q. Q., Gardner, B. B., Earley, J. U., Molkentin, J. D., McNally, E. M. (2014) Excess SMAD signaling contributes to heart and muscle dysfunction in muscular dystrophy. Hum. Mol. Genet. 23, 6722–6731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morales, M. G., Acuña, M. J., Cabrera, D., Goldschmeding, R., Brandan, E. (2018) The pro-fibrotic connective tissue growth factor (CTGF/CCN2) correlates with the number of necrotic-regenerative foci in dystrophic muscle. J. Cell Commun. Signal. 12, 413–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Córdova, G., Rochard, A., Riquelme-Guzmán, C., Cofré, C., Scherman, D., Bigey, P., Brandan, E. (2015) SMAD3 and SP1/SP3 transcription factors collaborate to regulate connective tissue growth factor gene expression in myoblasts in response to transforming growth factor β. J. Cell. Biochem. 116, 1880–1887 [DOI] [PubMed] [Google Scholar]
- 19.Mezzano, V., Cabrera, D., Vial, C., Brandan, E. (2007) Constitutively activated dystrophic muscle fibroblasts show a paradoxical response to TGF-beta and CTGF/CCN2. J. Cell Commun. Signal. 1, 205–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vial, C., Zúñiga, L. M., Cabello-Verrugio, C., Cañón, P., Fadic, R., Brandan, E. (2008) Skeletal muscle cells express the profibrotic cytokine connective tissue growth factor (CTGF/CCN2), which induces their dedifferentiation. J. Cell. Physiol. 215, 410–421 [DOI] [PubMed] [Google Scholar]
- 21.Morales, M. G., Gutierrez, J., Cabello-Verrugio, C., Cabrera, D., Lipson, K. E., Goldschmeding, R., Brandan, E. (2013) Reducing CTGF/CCN2 slows down mdx muscle dystrophy and improves cell therapy. Hum. Mol. Genet. 22, 4938–4951 [DOI] [PubMed] [Google Scholar]
- 22.Liu, S., Shi-wen, X., Abraham, D. J., Leask, A. (2011) CCN2 is required for bleomycin-induced skin fibrosis in mice. Arthritis Rheum. 63, 239–246 [DOI] [PubMed] [Google Scholar]
- 23.Kanisicak, O., Mendez, J. J., Yamamoto, S., Yamamoto, M., Goldhamer, D. J. (2009) Progenitors of skeletal muscle satellite cells express the muscle determination gene, MyoD. Dev. Biol. 332, 131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanisicak, O., Khalil, H., Ivey, M. J., Karch, J., Maliken, B. D., Correll, R. N., Brody, M. J., J Lin, S. C., Aronow, B. J., Tallquist, M. D., Molkentin, J. D. (2016) Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 7, 12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hack, A. A., Lam, M. Y., Cordier, L., Shoturma, D. I., Ly, C. T., Hadhazy, M. A., Hadhazy, M. R., Sweeney, H. L., McNally, E. M. (2000) Differential requirement for individual sarcoglycans and dystrophin in the assembly and function of the dystrophin-glycoprotein complex. J. Cell Sci. 113, 2535–2544 [DOI] [PubMed] [Google Scholar]
- 26.Tinsley, J. M., Potter, A. C., Phelps, S. R., Fisher, R., Trickett, J. I., Davies, K. E. (1996) Amelioration of the dystrophic phenotype of mdx mice using a truncated utrophin transgene. Nature 384, 349–353 [DOI] [PubMed] [Google Scholar]
- 27.Accornero, F., van Berlo, J. H., Benard, M. J., Lorenz, J. N., Carmeliet, P., Molkentin, J. D. (2011) Placental growth factor regulates cardiac adaptation and hypertrophy through a paracrine mechanism. Circ. Res. 109, 272–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi, Y. M., Rader, E. P., Crawford, R. W., Iyengar, N. K., Thedens, D. R., Faulkner, J. A., Parikh, S. V., Weiss, R. M., Chamberlain, J. S., Moore, S. A., Campbell, K. P. (2008) Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature 456, 511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruifrok, A. C., Johnston, D. A. (2001) Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol. 23, 291–299 [PubMed] [Google Scholar]
- 30.Petrosino, J. M., Heiss, V. J., Maurya, S. K., Kalyanasundaram, A., Periasamy, M., LaFountain, R. A., Wilson, J. M., Simonetti, O. P., Ziouzenkova, O. (2016) Graded maximal exercise testing to assess mouse cardio-metabolic phenotypes. PLoS One 11, e0148010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durbeej, M., Campbell, K. P. (2002) Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr. Opin. Genet. Dev. 12, 349–361 [DOI] [PubMed] [Google Scholar]
- 32.Whittaker, P., Kloner, R. A., Boughner, D. R., Pickering, J. G. (1994) Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res. Cardiol. 89, 397–410 [DOI] [PubMed] [Google Scholar]
- 33.Ivey, M. J., Tallquist, M. D. (2016) Defining the cardiac fibroblast. Circ. J. 80, 2269–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leask, A., Abraham, D. J. (2003) The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem. Cell Biol. 81, 355–363 [DOI] [PubMed] [Google Scholar]
- 35.Leask, A. (2013) CCN2: a novel, specific and valid target for anti-fibrotic drug intervention. Expert Opin. Ther. Targets 17, 1067–1071 [DOI] [PubMed] [Google Scholar]
- 36.Accornero, F., van Berlo, J. H., Correll, R. N., Elrod, J. W., Sargent, M. A., York, A., Rabinowitz, J. E., Leask, A., Molkentin, J. D. (2015) Genetic analysis of connective tissue growth factor as an effector of transforming growth factor β signaling and cardiac remodeling. Mol. Cell. Biol. 35, 2154–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gravning, J., Ahmed, M. S., von Lueder, T. G., Edvardsen, T., Attramadal, H. (2013) CCN2/CTGF attenuates myocardial hypertrophy and cardiac dysfunction upon chronic pressure-overload. Int. J. Cardiol. 168, 2049–2056 [DOI] [PubMed] [Google Scholar]
- 38.Fontes, M. S., Kessler, E. L., van Stuijvenberg, L., Brans, M. A., Falke, L. L., Kok, B., Leask, A., van Rijen, H. V., Vos, M. A., Goldschmeding, R., van Veen, T. A., et al. 2015) CTGF knockout does not affect cardiac hypertrophy and fibrosis formation upon chronic pressure overload. J. Mol. Cell Cardiol. 88, 82–90 [DOI] [PubMed] [Google Scholar]
- 39.Takagi, R., Ogasawara, R., Tsutaki, A., Nakazato, K., Ishii, N. (2016) Regional adaptation of collagen in skeletal muscle to repeated bouts of strenuous eccentric exercise. Pflugers Arch. 468, 1565–1572 [DOI] [PubMed] [Google Scholar]
- 40.Tierney, M. T., Gromova, A., Sesillo, F. B., Sala, D., Spenlé, C., Orend, G., Sacco, A. (2016) Autonomous extracellular matrix remodeling controls a progressive adaptation in muscle stem cell regenerative capacity during development. Cell Rep. 14, 1940–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemos, D. R., Babaeijandaghi, F., Low, M., Chang, C. K., Lee, S. T., Fiore, D., Zhang, R. H., Natarajan, A., Nedospasov, S. A., Rossi, F. M. (2015) Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat. Med. 21, 786–794 [DOI] [PubMed] [Google Scholar]
- 42.Calve, S., Odelberg, S. J., Simon, H. G. (2010) A transitional extracellular matrix instructs cell behavior during muscle regeneration. Dev. Biol. 344, 259–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith, L. R., Barton, E. R. (2018) Regulation of fibrosis in muscular dystrophy. Matrix Biol. 68-69, 602–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quattrocelli, M., Spencer, M. J., McNally, E. M. (2017) Outside in: the matrix as a modifier of muscular dystrophy. Biochim. Biophys. Acta 1864, 572–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fallon, J. R., McNally, E. M. (2018) Non-glycanated biglycan and LTBP4: leveraging the extracellular matrix for Duchenne muscular dystrophy therapeutics. Matrix Biol. 68-69, 616–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Acuna, M. J., Brandan, E. (2017) Analysis of pathological activities of CCN2/CTGF in muscle dystrophy. Methods Mol. Biol. 1489, 513–521 [DOI] [PubMed] [Google Scholar]
- 47.Sun, G., Haginoya, K., Wu, Y., Chiba, Y., Nakanishi, T., Onuma, A., Sato, Y., Takigawa, M., Iinuma, K., Tsuchiya, S. (2008) Connective tissue growth factor is overexpressed in muscles of human muscular dystrophy. J. Neurol. Sci. 267, 48–56 [DOI] [PubMed] [Google Scholar]
- 48.Song, Y., Yao, S., Liu, Y., Long, L., Yang, H., Li, Q., Liang, J., Li, X., Lu, Y., Zhu, H., Zhang, N. (2017) Expression levels of TGF-β1 and CTGF are associated with the severity of Duchenne muscular dystrophy. Exp. Ther. Med. 13, 1209–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carmignac, V., Durbeej, M. (2012) Cell-matrix interactions in muscle disease. J. Pathol. 226, 200–218 [DOI] [PubMed] [Google Scholar]
- 50.Thomas, K., Engler, A. J., Meyer, G. A. (2015) Extracellular matrix regulation in the muscle satellite cell niche. Connect. Tissue Res. 56, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Relaix, F., Zammit, P. S. (2012) Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development 139, 2845–2856 [DOI] [PubMed] [Google Scholar]
- 52.González, M. N., de Mello, W., Butler-Browne, G. S., Silva-Barbosa, S. D., Mouly, V., Savino, W., Riederer, I. (2017) HGF potentiates extracellular matrix-driven migration of human myoblasts: involvement of matrix metalloproteinases and MAPK/ERK pathway. Skelet. Muscle 7, 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith, L. R., Hammers, D. W., Sweeney, H. L., Barton, E. R. (2016) Increased collagen cross-linking is a signature of dystrophin-deficient muscle. Muscle Nerve 54, 71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ricard-Blum, S., Ville, G. (1989) Collagen cross-linking. Int. J. Biochem. 21, 1185–1189 [DOI] [PubMed] [Google Scholar]
- 55.Kutchuk, L., Laitala, A., Soueid-Bomgarten, S., Shentzer, P., Rosendahl, A. H., Eilot, S., Grossman, M., Sagi, I., Sormunen, R., Myllyharju, J., Mäki, J. M., Hasson, P. (2015) Muscle composition is regulated by a Lox-TGFβ feedback loop. Development 142, 983–993 [DOI] [PubMed] [Google Scholar]
- 56.Csiszar, K. (2001) Lysyl oxidases: a novel multifunctional amine oxidase family. Prog. Nucleic Acid Res. Mol. Biol. 70, 1–32 [DOI] [PubMed] [Google Scholar]
- 57.Smith, L. R., Barton, E. R. (2014) Collagen content does not alter the passive mechanical properties of fibrotic skeletal muscle in mdx mice. Am. J. Physiol. Cell Physiol. 306, C889–C898 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.