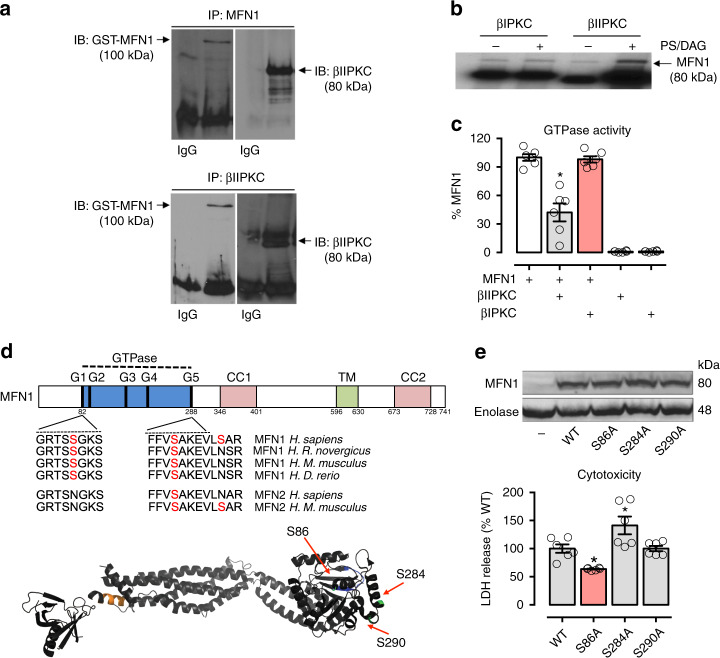
Fig. 4.
Characterization of Mfn1-βIIPKC protein–protein interaction. Recombinant Mfn1 (GST-Mfn1, 25 ng) was incubated with recombinant βIIPKC (GST-βIIPKC, 25 ng) in the presence and absence of phosphatidylserine and diacylglycerol (PS/DAG/Ca2+, PKC activators) for 30 min at 37 °C. a Co-immunoprecipitates with anti-Mfn1 and βIIPKC were analyzed by western blot. b Mfn1 phosphorylation was evaluated using radioactive [32P] 32P-ATP incorporation after incubation with either βIIPKC or its alternative splicing βIPKC in the presence and absence of classic PKC activators. Representative blots of three independent experiments. c GTPase activity of Mfn1 determined in the presence of active βIIPKC or its alternative splicing βIPKC (n = 6 per group). d Conservation of the βIIPKC phosphorylation in human orthologs of Mfn1 in rat, mouse, and zebrafish and Mfn2 in mouse. Ribbon presentation of the 3D structure of Mfn1 phosphorylation at the βIIPKC site (PDB: 5GOM). e Individual replacement of serine by alanine at residues 86, 284, and 290 in Mfn1 knockout MEFs. Cells were transfected with the following constructs: WT, S86A, S284A and S290A. 48 h after transfection cells were incubated with H2O2 (100 μM, 24 h) and measured cytotoxicity (LDH release, n = 6 per group). Data are means ± SEM. *P < 0.05 vs. control. One-way analysis of variance (ANOVA) with post-hoc testing by Duncan