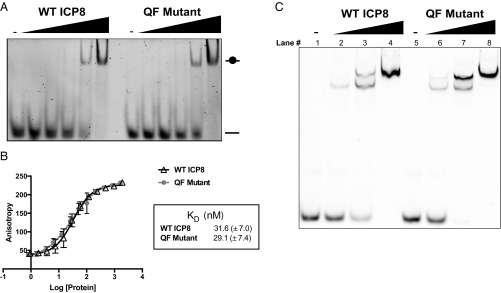
Fig. 3.
The QF mutant binds to ssDNA. (A) EMSA showing the ability of WT ICP8 and QF mutant proteins to bind to ssDNA. Proteins were titrated (0–300 nM, twofold serial dilution) against 100 nM fluorescein-labeled dT-14-mer ssDNA oligo in DNA binding buffer. Samples were incubated for 30 min at room temperature. Bound and unbound species were separated on 5% nondenaturing polyacrylamide gel in 1× TBE and imaged using a fluorescence imager. (B) Purified WT or QF mutant proteins were titrated (∼0.9–2 µM) against 2.5 nM of the same fluorescein-labeled dT-14-mer in the DNA binding buffer used above. FP measurements were taken on TECAN M1000 Pro plate reader with excitation wavelength of 470 nm and emission wavelength of 521 nm. Triplicate readings were averaged and the data from three independent experiments were fit to a 1:1 binding model from which dissociation constants (Kd) were determined. (C) A gel shift assay was performed with a Cy3-labeled 50-mer capable of binding multiple ICP8 monomers. Proteins were titrated (0, 50, 200, 400 nM) against 100 nM oligo.