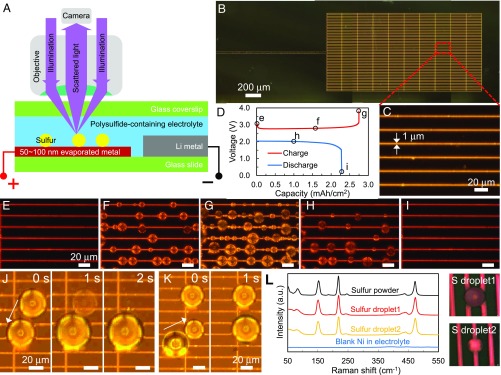
Fig. 1.
In operando observation of supercooled sulfur generated electrochemically at room temperature. (A) Schematic of the electrochemical cell design that allows in operando DFLM observation. (B and C) DFLM images of the nickel metal grid (50 nm thick, 1 μm wide) fabricated on glass slide as a substrate for the electrochemical cell. Bright lines are nickel, elsewhere is glass. (D–I) Voltage profile of the cell (D) and corresponding time-lapse DFLM images (E–I) showing the formation and dissolution of supercooled sulfur droplets. (J and K) Two sets of time-lapse images showing rapid merging of neighboring droplets and relaxation to spherical shape within 1 s, indicating the liquid nature of sulfur. (L) In situ Raman spectra of supercooled sulfur droplets. (Right) Corresponding bright-field light microscopy images captured by the Raman microscope are shown (magnification: 50×). The spectra match that of solid S8 powder, and the signals are not from electrolyte or substrate.