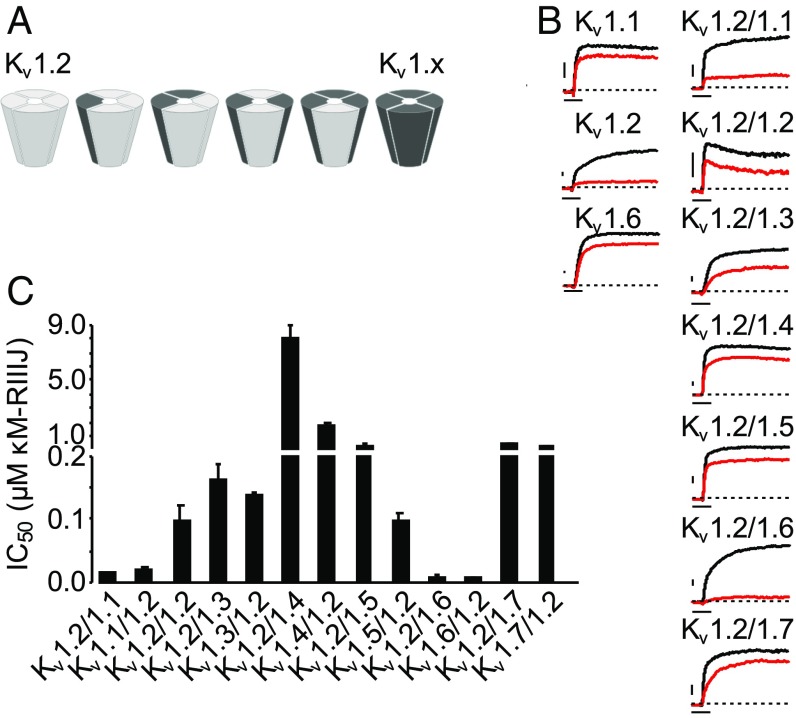
Fig. 1.
κM-RIIIJ blocks homomeric and heteromeric Kv1 channels. (A) Schematic representation of all possible combinations between two different Kv1 subunits. Homomeric and heteromeric channels containing Kv1.2 α-subunits and a second other Kv1 family (Kv1.x) subunit are displayed. Similar combinations are possible for all Kv1.x subunits with up to four different subunits forming a functional channel. (B) κM-RIIIJ block of homo- and heterodimeric Kv1 channels. Current traces in control (black) and in the presence of κM-RIIIJ (red) are shown. (Left) At 1 µM, κM-RIIIJ blocks homomeric Kv1.1 and Kv1.6 with low apparent affinity (IC50 ∼ 3–5 µM), whereas it displays ∼10× higher potency for Kv1.2 homomers (IC50 ∼ 300 nM). (Right) Representative dimeric channel-mediated currents in control and 100 nM κM-RIIIJ evidencing stronger block of Kv1.2/1.1 and Kv1.2/1.6 channels. Due to extreme differences in apparent affinity, 50 nM κM-RIIIJ is shown for Kv1.2/1.1 and 2 μM for Kv1.2/1.4. Dashed lines indicate zero current. (Scale bars, 500 pA and 10 ms.) (C) κM-RIIIJ affinity toward Kv1.2-containing dimeric Kv1 channels. The bar diagram presented summarizes κM-RIIIJ IC50s for dimeric constructs in forward or reversed order. In comparison with homomeric Kv1.2 channels, κM-RIIIJ has a 10- to 20-fold higher affinity for heterodimeric channels composed of Kv1.2 and either Kv1.1 or Kv1.6 subunits. All other dimeric constructs are blocked similarly or less strongly than homomeric Kv1.2. The order of the subunits in the concatemers bears no major influence on κM-RIIIJ’s activity.