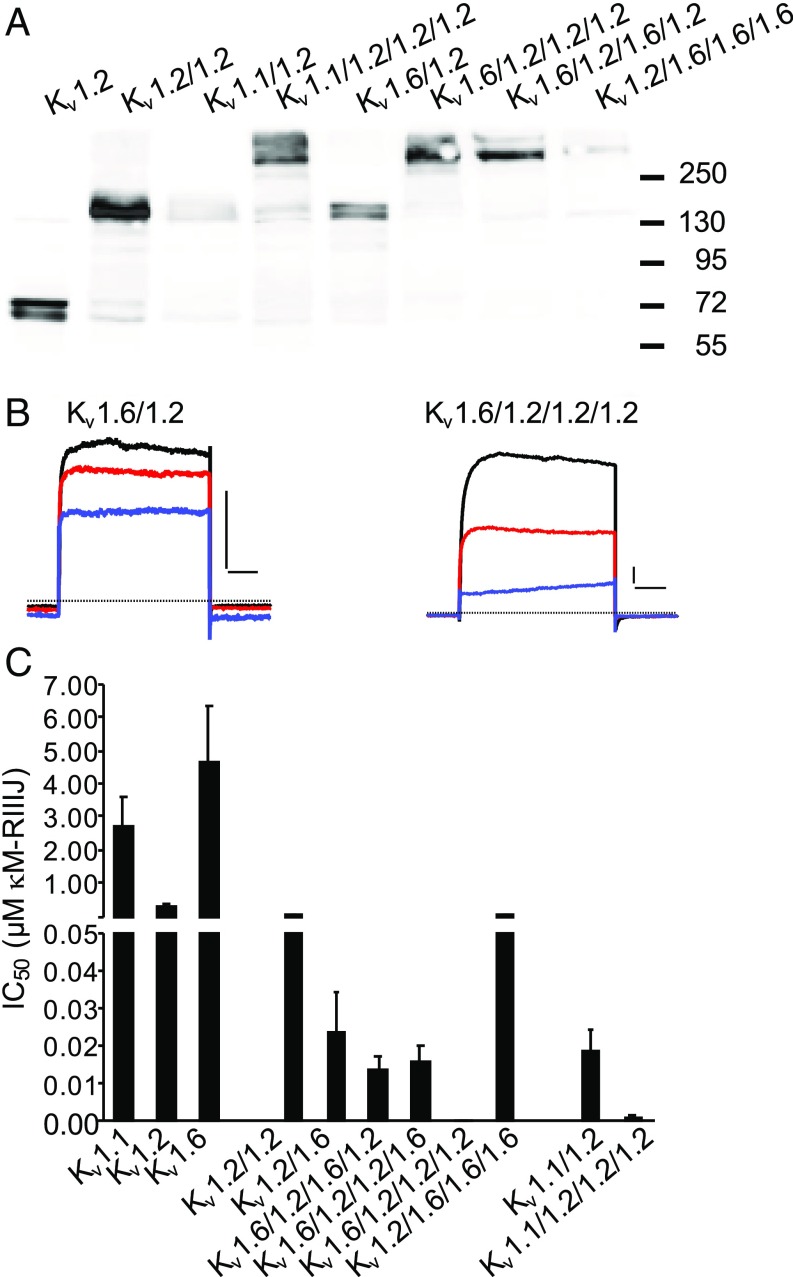
Fig. 2.
κM-RIIIJ displays enhanced apparent affinity toward asymmetric Kv1 channels. (A) Western blot analysis of selected concatemeric constructs expressed in HEK293. Bands corresponding to 75-, 150-, and 300-kDa protein products were recognized by an anti-Kv1.2 antibody evidencing expression of monomeric, dimeric, and tetrameric channels, respectively. (B) Representative current traces of symmetric heterodimeric and asymmetric heterotetrameric channels composed of Kv1.2 and Kv1.6. The effect of 0.5 nM (red) and 5 nM (blue) κM-RIIIJ on evoked K+ currents from Kv1.6/1.2 dimer and tetrameric concatemers composed of three Kv1.2 subunits and one Kv1.6 subunit are shown (black: control). The zero current level is marked by the dashed line. (Scale bars, 1 nA and 50 ms.) (C) IC50s of κM-RIIIJ block of homomeric and heteroconcatemeric Kv1 channels formed by Kv1.1, Kv1.2, and Kv1.6 subunits in different combinations. κM-RIIIJ displayed extremely high apparent affinity (sub-nM) for asymmetric concatemers composed of one Kv1.1 or Kv1.6 subunit together with three Kv1.2 subunits (SI Appendix, Table S2).