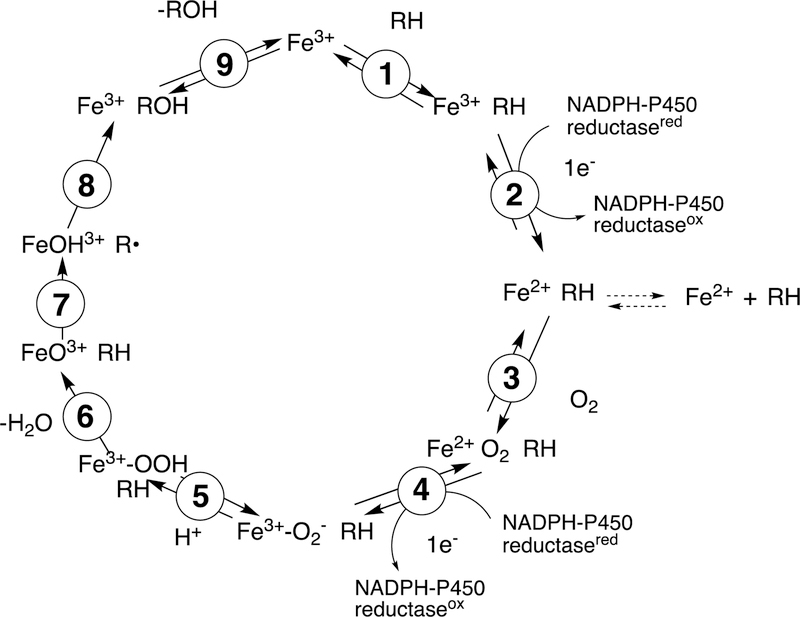
Figure 2.
General catalytic cycle for P450 reactions. RH indicates a substrate. The electrons are provided by either NADPH-P450 reductase (microsomal P450s, as shown) or a ferredoxin reductase/ferredoxin system (mitochondrial and some bacterial P450s). For some other electron delivery modes see Guengerich and Munro.31 Note the Fe3+–O2− (ferric peroxide, Compound 0) and FeO3+ (Compound I) forms discussed in the text. The electron transfers from the reductase are simplifications in that the course of electron flow is probably from FMNH2/FADH• to FMNH•/FADH• in the first reduction (step 2) and (assuming that the reductase contributes the second electron to the P450) from FMNH•/FAD• to FMNH•/FAD in the second reduction step (4). In many bacterial systems and in mammalian mitochondria the electrons are donated by ferredoxins (e.g., adrenodoxin, putidaredoxin). In some cases with mammalian P450s, the second electron (step 4) is donated by cytochrome b5 (b5).32 In the literature there exists different nomenclature for the same iron intermediates in this P450 catalytic cycle.33,34 For clarity, throughout the text of this manuscript Compound I is referred to interchangeably with FeO3+, and ferric peroxide is referred to interchangeably with FeIII–O2− or Compound 0 (protonated form FeIII–OOH).