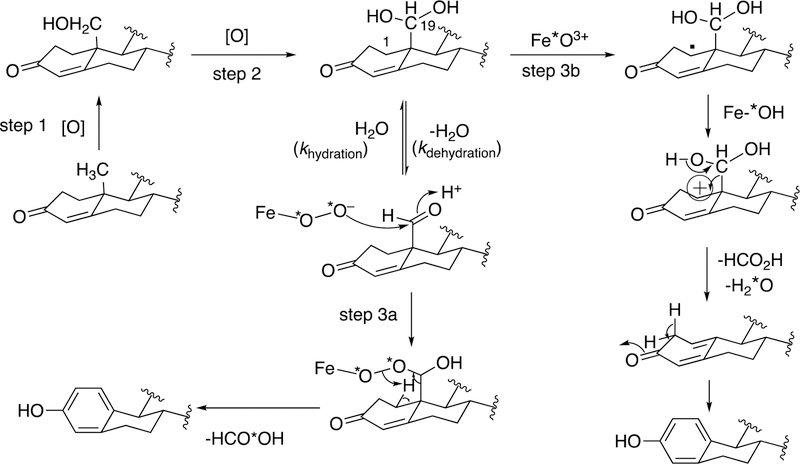
Figure 58.
Use of 18O labeling studies in defining the mechanism of P450 19A1.177,220,222,223 Steps 1 and 2 are generally agreed to involve the P450 FeO3+ entity and hydrogen atom abstraction/oxygen rebound.224 Two possibilities are shown for Step 3 in the presence of 18O2. In Step 3a, the FeIII–O2− entity participates in a nucleophilic attack on the 19-aldehyde. In Step 3b, the FeO3+ form of the P450 19A1 abstracts the 1β-hydrogen atom of the gem-diol. Electron transfer yields the carbocation, which collapses to yield the estrogen product. In Step 3a, the HCO2H must contain label (18O) but not in Step 3b. “*O” denotes 18O. The step 3(b) pathway is supported by recent evidence.177