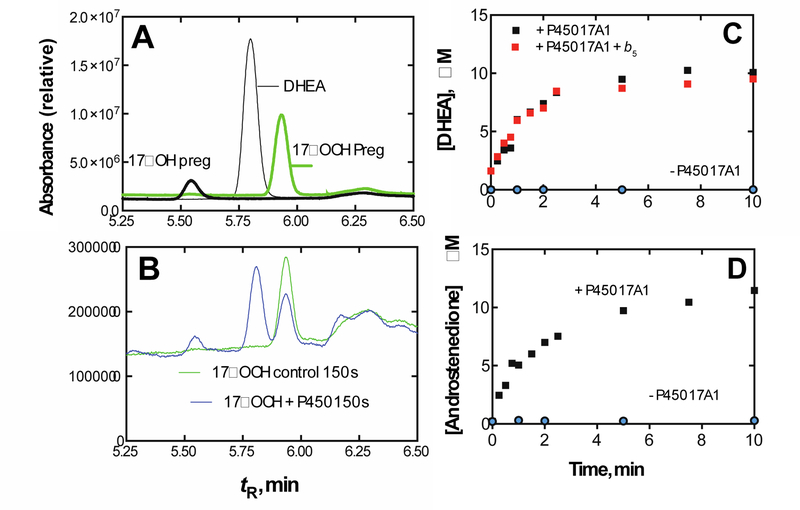
Figure 66.
Conversion of 17α-hydroperoxypregnenolone to lyase products by human P450 17A1.239,256 (Reprinted with permission from J. Biol. Chem. 2018, 293, 541–556, Figure 8) (A) HPLC of standard 17α-hydroperoxypregnenolone, 17α-hydroxypregnenolone, and DHEA, showing tR for elution. (B) Chromatograms of reaction of 25 µM 17α-hydroperoxypregnenolone without (green) or with (blue) 60 nM human P450 17A1 after 150 s. Absorbance (relative units) was integrated over the UV range (200–350 nm). See ref. 239 for other details regarding chromatography. (C) Time course of conversion of 17α-hydroperoxypregnenolone to DHEA, ± human b5. (D) Time course of conversion of 17α-hydroperoxyprogesterone to androstenedione.