Abstract
Brain plasticity has often been quoted as a reason for the more favorable outcome in childhood stroke compared to adult stroke. We investigated the relationship between language abilities and language localization in childhood stroke. Seventeen children and adolescents with left- or right-sided ischemic stroke and 18 healthy controls were tested with a comprehensive neurolinguistic test battery, and the individual neural representation of language was measured with an fMRI language paradigm. Overall, 12 of 17 stroke patients showed language abilities below average, and five patients exhibited impaired language performance. fMRI revealed increased activity in right hemisphere areas homotopic to left hemisphere language regions. In sum, seven stroke patients revealed atypical, i.e. bilateral or right lateralized language representation. Typical left hemispheric language lateralization was associated with better performance in naming and word fluency, whereas increased involvement of right homologues was accompanied by worse language outcome. In contrast, lesion lateralization or lesion volume did not correlate with language outcome or atypical language lateralization. Thus, atypical language lateralization is unfavorable for language outcome, and right homologues do not have the same cognitive capacity, even in young children.
Keywords: language, cognitive function, childhood stroke, functional neuroimaging
1. Introduction
Childhood stroke is emerging as a serious disorder with increasing incidence in the last decade. 1 It is defined as a cerebrovascular event that occurs between 30 days and 18 years of age. 2 Ischemic stroke represents half of all childhood strokes with an incidence rate of 2-5 / 100.000 children per year. 3 The territory of the middle cerebral artery is most commonly affected in childhood ischemic stroke, thus, hemiparesis and cognitive deficits are the most common acute clinical presentations of this disorder. 4, 5 Although prognosis for recovery after stroke in childhood is better than in adults, studies have found residual cognitive impairments in more than half of the children who have experienced a stroke. 2, 3 Studies focusing on language functioning in childhood stroke report frequent word finding deficits, reduced discourse abilities, deficits in written language acquisition, and syntactic impairments. 6–9 Surprisingly, these language deficits do not only occur after left-hemisphere stroke in childhood, but may also be observed following right-hemisphere childhood stroke, 7, 9 thus supporting that the theoretical view of language-brain mapping derived from adult studies does not apply to children’s brains. A specific period of vulnerability for cognitive deficits following cortical ischemic stroke has been reported for the age of one month to five years, a time span corresponding exactly to the period of primary language acquisition. 10
Brain plasticity has often been quoted as the reason for the more favorable outcome in childhood stroke relative to adult stroke. However, the relationship between the individual capacity for neural modification and cognitive functioning is unclear. 11 In children with perinatal focal brain injury, left anterior and bilateral posterior language lateralization was associated with better language functioning. 12 However, differences between perinatal and childhood stroke regarding pathologies and cognitive outcome are well known. 13 These findings may thus not apply to children with strokes occuring during childhood, the dynamic phase of language acquisition and consolidation. Two studies investigated the relationship between behavioral language abilities and cerebral language organization in left-sided childhood stroke and found contradictory results: Elkana et al. examined seven children and young adults with left focal brain lesions and found greater right hemisphere lateralization in patients compared to controls; however, increased performance in linguistic tasks was associated with greater lateralization to the left hemisphere. 14 Lidzba et al. investigated twelve patients (five children) who experienced a left hemisphere stroke in childhood with language fMRI and questionnaires for self-assessment of language problems. 15 Language lateralization towards the right hemisphere, which occurred only in the younger participants, was found to be favorable for language outcome in their study. In contrast, no study exists on the relationship between language abilities and functional language localization after right-sided childhood stroke.
In summary, previous functional imaging studies on the brain-behavior relationship in children with left-sided stroke indicate that language localization often takes place in networks in which preserved left or homotopic right hemisphere areas might compensate for damage. Yet, it remains unclear whether atypical language lateralization is advantageous or unfavorable for language functioning in children suffering from focal brain injury. In addition, the relationship between language abilities and language localization in children with right-sided stroke is unknown. To answer these questions, we examined 17 patients with unilateral left- or right-sided childhood stroke and 18 age-matched healthy controls with an extensive language test battery and fMRI for language localization to explore the plasticity of the child’s brain and its relationship to language functioning.
2. Materials and methods
2.1. Participants
Seventeen children with unilateral ischemic stroke, aged seven to 17 years, were recruited at the neuropediatric outpatient unit of the Department of Pediatrics and Adolescent Medicine, Medical University of Vienna. All patients had suffered from unilateral focal brain damage due to ischemic stroke after the first month of life. Exclusion criteria were an active seizure disorder, antiepileptic medication, or developmental problems prior to stroke. Table 1 shows characteristics of study participants.
Table 1. Patient demographics and lesion information.
| At stroke presentation | At study | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Sex | Age at stroke (y;m) | Vascular territory | Infarct location | Lesion side | modASPECTS | Symptomes | Time between stroke and study (y;m) | Age at study (y;m) | EHI Score | Residual lesion volume cm3 |
| 1 | f | 6;9 | MCA | basal ganglia | left | 1 | seizure, hemiparesis | 1;5 | 8;2 | 0.90 | 0.78 |
| 2 | m | 1;4 | MCA | basal ganglia | left | 2 | seizure, hemiparesis | 9;0 | 10;4 | 0.90 | 15.19 |
| 3 | f | 8;9 | MCA | cortical, subcortical, basal ganglia | left | 5 | headache, hemiparesis | 7;5 | 16;4 | -1.00 | 43.46 |
| 4 | m | 5;5 | PCA | cortical, subcortical, thalamus | left | 2 | neck ache, headache, aLOC, ataxia, vomiting, hemiparesis, dysarthria | 2;0 | 7;5 | 0.80 | 71.42 |
| 5 | m | 6;2 | MCA | cortical, subcortical, basal ganglia | left | 7 | aphasia, vomiting, hemiparesis | 0;8 | 7;0 | -0.56 | 43.94 |
| 6 | f | 16;7 | MCA | cortical (insula) | left | 1 | aphasia, hemiparesis | 0;10 | 17;5 | 1.00 | 0.24 |
| 7 | m | 11;11 | PCA | cortical, subcortical, thalamus | left | 3 | nausea, dizziness, aLOC, seizures, hemianopsia | 0;3 | 12;2 | 1.00 | 9.26 |
| 8 | f | 11;10 | PCA | cortical, subcortical, thalamus | left | 2 | headache, visual disturbances, altered mental state | 0;7 | 12;5 | 0.50 | 3.35 |
| 9 | m | 12;10 | MCA | cortical, basal ganglia | left | 4 | aphasia, hemiparesis | 0;8 | 13;6 | 0.80 | 0.51 |
| 10 | f | 10;1 | MCA | cortical, subcortical, basal ganglia | right | 9 | aLOC, hemiparesis | 6;11 | 17;0 | 1.00 | 331.53 |
| 11 | m | 10;2 | MCA | cortical, subcortical | right | 5 | seizures | 2;10 | 13;0 | 1.00 | 0.09 |
| 12 | m | 9;1 | MCA | cortical, subcortical, basal ganglia | right | 8 | hemiparesis, aLOC | 0;3 | 9;4 | 1.00 | 264.42 |
| 13 | m | 14;6 | MCA | cortical, subcortical, basal ganglia, thalamus | right | 8 | headache, seizures, hemiparesis, aLOC, aphasia | 0;5 | 14;11 | 0.50 | 146.72 |
| 14 | m | 5;0 | MCA | subcortical, basal ganglia | right | 3 | hemiparesis | 10;2 | 15;2 | 0.70 | 19.41 |
| 15 | m | 12;3 | MCA | basal ganglia | right | 4 | aLOC, hemiparesis | 4;8 | 16;11 | 1.00 | 6.15 |
| 16 | m | 8;6 | MCA | cortical, subcortical, basal ganglia | right | 9 | seizure, aLOC, hemiparesis | 5;9 | 14;3 | 0.60 | 49.45 |
| 17 | m | 13;2 | PCA | thalamus | right | 1 | headache, visual disturbance, ataxia | 1;4 | 14;6 | 0.70 | 0.17 |
Note: y = years, m = months, modASPECTS = modified pediatric version of the Alberta Stroke Program Early Computed Tomography Score, MCA = middle cerebral artery, PCA = posterior cerebral artery, aLOC = abnormal level of consciousness, EHI = Edinburgh Handedness Inventory
In addition, 18 healthy, right-handed children matched for gender and age at the time of testing were included as controls in the study. The controls had no history of neurological disease or clinical evidence of neurological dysfunction or developmental delay. They were recruited by blackboard announcement and flier distribution.
Further inclusion criteria for all study participants were native German speaking, normal hearing, normal or corrected-to-normal vision, sufficient language comprehension (age-corrected z-value in the Token Test for Children above SD -2.00), 16 and no MRI contraindications. All study participants received a 30 € voucher for a book store. Written informed consent was obtained from a parent or legal representative in all cases. Furthermore, age-adjusted written information was given to the children prior to the study, and all children gave verbal and written assent. This study was approved by the Ethics Committee of the Medical University of Vienna and performed in accordance with the Helsinki Declaration of 1975.
2.2. Cognitive Assessment
Verbal abilities were assessed using standardized tests which examine functions important for language development and consolidation. 17 Expressive vocabulary was tested using the Wortschatz- und Wortfindungstest which requires the naming of visually presented objects, situations, and conditions. 18 Language comprehension was measured with the Token Test for children where tokens varying in size and shape have to be moved according to auditory commands with increasing length and linguistic complexity. 16 The TROG-D was used to examine grammatical comprehension of sentences of increasing morphosyntactic complexity. 19 Common word, pseudoword, and text reading fluency and accuracy were tested using the Salzburger Lese- und Rechtschreibtest SLRT II and the Zürcher Lesetest ZLT II. 20–21 Spelling accuracy was investigated with the SLRT. Categorial word fluency was evaluated using the Regensburger Wortflüssigkeitstest which requires the participant to name, within two minutes, as many words as possible of a semantic category (animals). 22 Verbal memory was assessed with the German version of the Auditory Verbal Learning Test, the Verbaler Lern- und Merkfähigkeitstest, which measures the learning efficiency of a words list, short-term recall after distraction, long-term recall, and recognition. 23–24
Raw scores of cognitive tests were transformed into age-adjusted z-scores for each test result. For the Wortschatz- und Wortfindungstest and the Salzburger Lese- und Rechtschreibtest, norms were only available up to 11 years of age, for the TROG-D up to 12 years, as acquisition of the verbal abilities tested with these measurements is assumed to be completed after this age. We therefore transformed the raw scores of these tests in elder participants into z-scores based on the norms of the eldest norm population with the risk of an overestimation of z-scores in these participants.
We clustered the test scores into six language abilities: naming (Wortschatz- und Wortfindungstest), comprehension (Token Test and Trog-D), reading (reading scores of the Salzburger Lese- und Rechtschreibtest and the Zürcher Lesetest), writing (writing scores of the Salzburger Lese- und Rechtschreibtest), word fluency (Regensburger Wortflüssigkeitstest), and verbal memory (Verbaler Lern- und Merkfähigkeitstest), and controlled this clustering with principal component analysis using SPSS Statistics (version 24.0). In line with clinical conventions, individual percentile ranks from SD -1.00 to SD 1.00 were defined within the average range. Performance below the SD -1.00 was read as below average, and performance below SD -2.00 was interpreted as impaired.
2.3. Handedness Assessment
Handedness was evaluated using the Edinburgh Handedness Inventory (EHI). 25 This questionnaire assesses the hand usage for commonly performed tasks such as cutting with a knive or drawing. The EHI Score is calculated by using the formula (Σright−Σleft)/(Σright+Σleft). Handedness was categorized as right if the EHI Score was ≥ 0.20, bilateral if within -0.20 and 0.20, or left if ≤ -0.20. This assessment was performed along with the cognitive assessment, in children with stroke the EHI score thus reflects post-stroke handedness.
2.4. MRI
2.4.1. FMRI paradigm
For functional language localization, the German version of an auditory description definition task was presented. This paradigm has shown robust language lateralization in healthy children and typically involves inferior and middle frontal regions, middle and superior temporal regions, as well as mesial temporal areas including the hippocampal formation. 26–30 In the auditory description definition condition, the participants listen to the definition of an object followed by a noun and are instructed to press a button each time the definition incorrectly described the noun. For instance, “A long yellow fruit is a banana.” (correct) or “Something you sit on is a spaghetti.” (incorrect). This paradigm was designed to elicit comprehension of a phrase, semantic recall, and semantic decision. Seventy percent of items are correct targets, and true and false descriptions are pseudo-randomly distributed. Performance in the scanner was monitored by the button-press. The control condition consisted of reverse speech, with a pure tone at the end of some items. The participants were instructed to press the button each time he/she heard the tone. Reversed speech has been demonstrated to avoid semantic processing in similar fMRI tasks, and the control condition was designed to control for first and second order auditory processing, attention, and motor response. 31–33 Task performance was evaluated by the overall accuracy in the language condition and the control task separately.
Three different, age-adjusted versions of the fMRI paradigm were available (7–9 years old, 10–12 years old, 13-17 years old). The difficulty levels were distinguished by manipulating linguistic criteria including word frequency, word length, and word complexity according to normative word data (www.wortschatz-unileipzig.de; accessed 09.09.2013). We used a block design composed of five language condition blocks alternating with five control task blocks. Each block lasted for 40 seconds and consisted of 10 sentences presented every four seconds. Total fMRI scanning time was 6 minutes 40 seconds.
2.4.2. MRI Image acquisition
All participants were scanned on a 3T Siemens TIM Trio scanner (Siemens Medical Solutions, Erlangen, Germany) equipped with a high-performance gradient system to support fast, high-resolution whole-brain echo-planar imaging. 3D structural MRI scans were performed using an isocubic magnetization-prepared rapid gradient-echo (MPRAGE, T1-weighted, TE/TR _ 4.21/2300ms, inversion time 900, with a matrix size of 240 x 256 x 160, voxel size 1 x 1 x 1.10 mm3, flip angle 9°) sequence. FMRI was acquired using a phase corrected blipped gradient echo, single shot echo planar imaging sequence. Altogether, 200 EPI volumes were acquired with a square FOV of 210 mm, voxel size 2.1 x 2.1 x 4 mm3, 25 percent gap and 20 slices aligned parallel to the AC-PC plane using a repetition time of 2000 ms, echo time 42 ms, and a flip angle of 90°.
2.4.3. MRI data analysis
For each patient, lesion masks were created. The manual demarcation of the brain lesions was performed by a trained rater on every single axial slice of the T1-weighted images using MRIcroGL Software (http://www.cabiatl.com/mricrogl; accessed 21.02.2017). These lesion masks were used to perform a cost function masking for normalization of brains. 34, 35 Furthermore, lesion masks were used for lesion volume calculation with MRIcron software (http://www.mricro.com/mricron; accessed 30.07.2017).
FMRI analyses were carried out using SPM8 (Wellcome Department of Cognitive Neurology, London, United Kingdom) implemented in MATLAB (Version 8.3 Mathworks, Inc., Sherborn, MA, U.S.A.). EPI volumes were spatially realigned and corrected for movement. Customized prior probability maps and a customized T1 template, matched to age and gender composition of the study group, were created by employing the Template-O-Matic toolbox. 36 Each subject’s anatomical image was segmented using the customized priors and the customized T1 template, then bias-corrected. This way the derived spatial normalization parameters were used to normalize the coregistered functional volumes. Normalized EPI volumes were smoothed using a Gaussian kernel with FWHM = 8 mm. BOLD signal increases pertaining to task-evoked responses in brain activity were modeled using a general linear model as implemented in SPM. Regressors modeling residual movement related variance (translational and rotational movement) were included in the model as covariates of no-interest.
Language activation was measured by contrasting the auditory description definition task condition > reversed language control condition. To examine the group effect of functional brain activations in healthy controls, random effects analysis (one-sample t test) was used, corrected for multiple comparisons family-wise error (FWE) with p < .05. For the group analyses of left hemisphere and right hemisphere stroke groups, respectively, fixed effects analyses were performed (pFWE < .05), due to the small number of subjects and the large heterogeneity of functional language localization. Thus, whereas the fMRI findings of the healthy control group are representative for the population the controls stem from, the fMRI results for the stroke groups are valid only for the investigated group of subjects. Single-subject fMRI activations were analyzed with a signficance threshold of puncorr < .001. Individual lateralization of activations was estimated at the single-subject level by use of the LI-toolbox. 37 In order to avoid the threshold dependency of lateralization indices (LIs), a bootstrapping approach was employed. LI was calculated according to the formula (Σactivationleft −Σactivationright)/(Σactivationleft)+Σactivationright) where “Σactivation” is the sum of activated voxels. The LI was categorized as left lateralized if ≥ 0.20, bilateral if within -0.20 and 0.20, or right if ≤ -0.20.
2.4.4. Retrospective analysis of initial MRI
The analysis of the MRI at stroke presentation was done retrospectively using the modified pediatric version of the Alberta Stroke Program Early Computed Tomography Score modASPECTS. 38 This modASPECTS was used to semiquantitatively assess infarct volume on axial diffusion-weighted MRI images in all supratentorial regions, including the territories of the anterior, middle, and posterior cerebral arteries as well as the thalamus. A region was scored positive if it was involved in the stroke area, yielding a maximum modASPECTS of 30.
2.5. Statistical analysis
Statistical analyses were conducted using SPSS Statistics (version 24.0). For normal distributed data (age at stroke, age at examination, time between stroke and examination, modAspects), two sample t-tests were used to examine group differences. Nonparametric testing was conducted whenever data were not normal distributed (handedness, residual lesion volume, language laterality indices, cognitive test results). Mann-Whitney U test was performed to investigate if cognitive test scores, residual lesion volumes, or laterality indices differed by sex or group, and χ2 test examined if groups differed by sex. Spearman’s rank correlation tested possible associations between these variables. Significance of correlations was set based on a strict Bonferroni correction factor, i.e. α = 0.05 / number of comparisons.
3. Results
3.1. Demographic and clinical differences between groups
Children with stroke and healthy controls did not differ significantly in age at examination or gender (Table 2). However, handedness was significantly less right in stroke patients compared to controls (U(n1 = 17, n2 = 18) = 72, p = 0.007, r = 0.51).
Table 2. Group differences of study participants.
| Left stroke group (n=9) |
Right stroke group (n=8) |
Patients (n=17) |
Controls (n=18) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Test value | p | Mean | SD | Mean | SD | Test value | p | |
| Age at examination | 11.63 | 3.76 | 14.39 | 2.44 | t = -1.76 | 0.099 | 12.93 | 3.41 | 10.93 | 2.93 | t = 1.87 | 0.071 |
| Handedness (EHI score) | 0.48 | 0.74 | 0.81 | 0.21 | U = 29 | 0.481 | 0.64 | 0.57 | 0.97 | 0.08 | U = 72 | 0.007 |
| Age at stroke (y) | 9.07 | 4.65 | 10.34 | 2.99 | t = -0.66 | 0.520 | ||||||
| Time between stroke and examination (y) | 2.52 | 3.31 | 4.00 | 3.50 | t = -0.90 | 0.384 | ||||||
| Lesion volume (cm3) | 20.91 | 25.78 | 102.24 | 131.25 | U = 27 | 0.423 | ||||||
| modASPECTS | 3.00 | 2.00 | 5.88 | 3.04 | t = -2.33 | 0.034 | ||||||
| Gender (f/m) | 4/5 | 1/7 | χ2 = 2.08 | 0.294 | 5/12 | 7/11 | χ2 = 0.35 | 0.724 | ||||
Note: Significance after Bonferroni correction is indicated with bold letters.
Abbreviation: modASPECTS = Alberta Stroke Program Early Computed Tomography Score; EHI = Edinburgh Handedness Inventory
The stroke group comprised of 9 children with left-sided and 8 children with right-sided stroke. Age at stroke, age at examination, and time between stroke and testing were not significantly different between the group of left- and the group of right-sided stroke (Table 2). Whereas gender was well-balanced in the left stroke group, there was only one girl in the right stroke group. However, this difference did not reach statistical significance. Furthermore, all children with right-sided stroke were right-handed, whereas two children with left-sided stroke were left-handed, but groups did not differ statistically significant in the strength of handedness as measured with the EHI. However, groups were significantly different regarding lesion volume: The initial modASPECTS, which semiquantetatively assessed stroke volume at stroke presentation, revealed a significantly higher infarct volume in the right stroke group compared to the left stroke group (t = -2.33, p = 0.034). Though the initial modASPECTS and residual lesion volumes at examination correlated significantly (rho = 0.66, p = 0.004), groups did not significantly differ in the residual lesion volumes at examination U(n1 = 9, n2 = 8) = 27, p = 0.423, r = 0.21).
3.2. Individual results
3.2.1. Individual language profiles
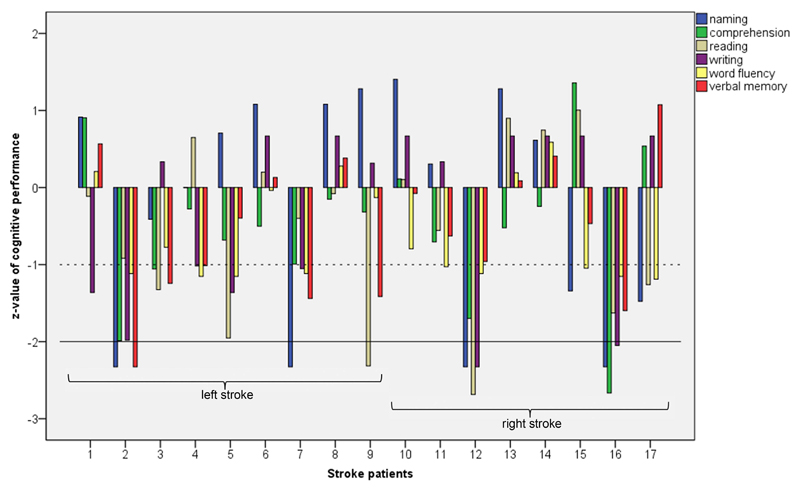
Figure 1 displays the individual language profiles in stroke patients. Overall, five out of 17 stroke patients (30 %) exhibited impaired language functions (SD -2.00). Three of them had language deficits in two or more language functions, two further children showed linguistic deficits in a single domain. Most often, naming abilities were compromised (n = 4), followed by reading and writing impairments (n = 2). One child showed profound verbal memory deficits, and one child had a formal language comprehension impairment. All these children showed further test scores below average (SD -1.00). Seven further stroke patients (41 %) revealed one or more language functions below average, with naming, word fluency, and written language being most often affected. Thus, when investigated in detail, 71 % of children with stroke (12/17) displayed below average or impaired language functions. Language deficits occurred in both left- and right-stroke groups.
Figure 1.
Individual language profiles in stroke patients. The solid line represents SD -2 (impaired function), the dashed line SD -1 (below average function).
In the healthy control group, most children performed within or above average in the language tests (Supplement Figure 1). However, five children were below average in one or more language functions. Three children showed below average abilities in writing, and one each in comprehension, verbal memory, and word fluency. None of the healthy controls exhibited impaired verbal functions. Overall, these results point to a wide distribution of language performances in the healthy study group.
3.2.2. Individual language localization and language laterality
On-site check of in-scanner performance showed adequate response during the fMRI paradigm in all participants. Unfortunately, due to technical reasons, task accuracy for the in-scanner performance was recorded only in 22 participants. Mean correct response in ten stroke patients was 96.34 % (SD 4.46) for the auditory description definition condition and 89.66 % (SD 13.47) for the tone condition. In 12 controls, mean correct response was 93.50 % (SD 8.58) for the auditory description definition task and 94.50 % (SD 7.29) for the tone task. Overall, these data indicate good task performances in both groups. However, due to the large amount of missing data, we waived further analyses of in-scanner task performances.
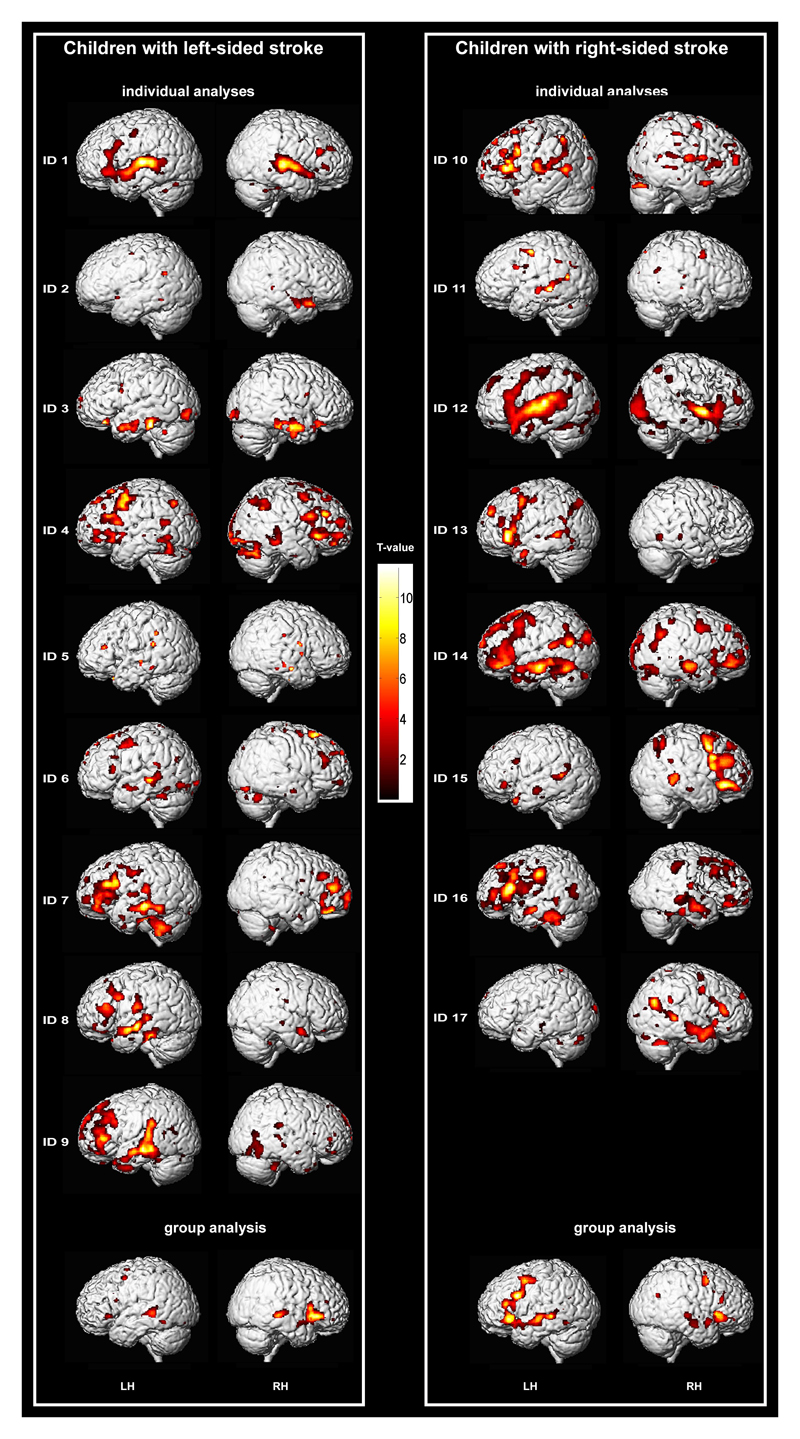
Figure 2 displays the individual language activations in each stroke patient. Overall, a large heterogeneity of localization, extent, and significance of activations can be observed. However, middle temporal and inferior and middle frontal regions were most consistently involved, either uni- or bilaterally.
Figure 2.
Individual language activations (puncorr < .001) on the individual 3D rendered brain from T1-weighted normalized images. Group activations (fixed effect, pFWE < .05) of the left stroke group and the right stroke group, respectively, on the 3D rendered brain of one participant. Left is left-hemisphere (LH).
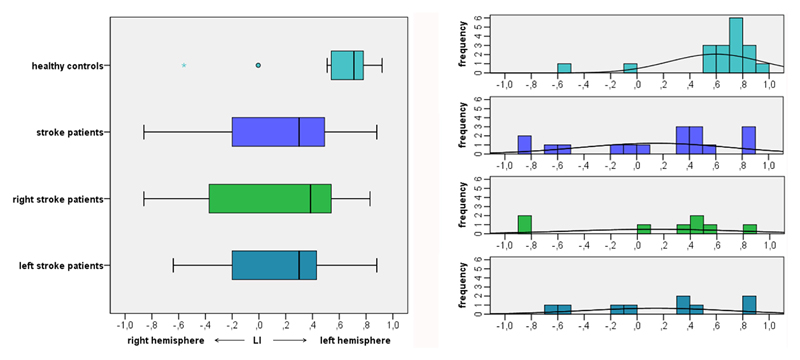
The calculation of individual language laterality revealed left lateralized language in ten children with stroke (Figure 3). Two patients with left-sided stroke and two children with right-sided stroke exhibited right lateralized language. Three further stroke patients had bilateral language representation, two with left, one with right stroke. Thus, overall, 50 % of left stroke patients and 33 % of right stroke patients showed atypical (i.e. bihemispheric or right) language lateralization.
Figure 3.
LIs in study participants. LIs are depicted in individual study participants and in the groups of healthy controls, stroke patients overall, right stroke patients, and left stroke patients. ° indicates asymmetric outliers, * indicates extreme outliers (farther than 3 interquartile ranges).
LIs in single subject analyses of healthy controls showed left-lateralized activations in 16/18 children. One healthy participant revealed bilateral language localization, one further healthy child exhibited right lateralized language. In sum, 11 % of healthy children showed atypical language lateralization.
3.3. Group results
3.3.1. Group language localization
In fMRI group analysis of the healthy control participants, one sample t-test revealed a typical language localization pattern with left lateralized activations in the inferior, middle, and medial frontal gyri, the insula, the middle temporal gyrus, the fusiform gyrus, and the hippocampus. In the right hemisphere, no activation was found on group level with a significance threshold of pFWE < 0.05 (Supplement Table 1, Supplement Figure 2).
In the left stroke group, fixed effect group analysis revealed left hemisphere activations in medial, middle, and inferior frontal regions, the insula, and the fusiform gyrus (Table 3, Figure 2). In addition, significant right hemisphere activations were found in inferior and middle frontal regions, the insula, and the superior temporal gyrus. The right stroke group exhibited left hemisphere activations in medial, middle, and inferior frontal regions, inferior, middle, and superior temporal areas, the angular gyrus, and mesial temporal areas including the hippocampus and the fusiform gyrus. Furthermore, right hemisphere activations were found in inferior and middle frontal areas, the insula, the superior temporal and the angular gyrus, and the fusiform gyrus.
Table 3. Group analyses of language activations in stroke patients.
| Anatomical region | MNI coordinates | Cluster size | T | ||
|---|---|---|---|---|---|
| x | y | z | |||
| LEFT STROKE GROUP | |||||
| Left hemisphere | |||||
| Medial frontal gyrus | -3 | 15 | 49 | 20109 | 10.01 |
| Insula/inferior frontal gyrus | -30 | 26 | -3 | 466 | 7.44 |
| Middle frontal gyrus | -48 | 3 | 51 | 124 | 6.77 |
| Fusiform gyrus | -50 | -52 | -12 | 95 | 6.03 |
| Middle frontal gyrus | -36 | 0 | 64 | 39 | 6.49 |
| Medial frontal gyrus | -3 | 39 | 39 | 59 | 5.34 |
| Inferior frontal gyrus | -51 | 15 | 18 | 40 | 5.46 |
| Inferior frontal gyrus | -53 | 20 | 28 | 4 | 4.85 |
| Inferior occipital gyrus | -27 | -85 | -14 | 5 | 4.83 |
| Middle frontal gyrus | -51 | 12 | 33 | 1 | 4.72 |
| Right hemisphere | |||||
| Inferior frontal gyrus/insula | 45 | 23 | -3 | 2965 | 8.74 |
| Superior temporal gyrus | 50 | -24 | 0 | 1131 | 8.62 |
| Middle frontal gyrus | 51 | 41 | 18 | 28 | 5.43 |
| Middle frontal gyrus | 29 | 51 | -9 | 18 | 5.08 |
| RIGHT STROKE GROUP | |||||
| Left hemisphere | |||||
| Medial frontal gyrus | -3 | 12 | 57 | 2409 | 10.88 |
| Precentral gyrus/inferior frontal gyrus /superior temporal gyrus | -50 | -3 | 51 | 6876 | 9.48 |
| Fusiform gyrus | -44 | -53 | -11 | 217 | 6.89 |
| Angular gyrus | -38 | -62 | 26 | 78 | 5.70 |
| Cuneus | -14 | -83 | 7 | 85 | 5.58 |
| Hippocampus | -18 | -7 | -15 | 17 | 5.46 |
| Inferior temporal lobe | -38 | -29 | -15 | 24 | 5.30 |
| Middle temporal lobe | -60 | -51 | 7 | 5 | 4.96 |
| Inferior temporal lobe | -39 | -12 | -26 | 1 | 4.84 |
| Cuneus | -5 | -83 | 15 | 1 | 4.75 |
| Right hemisphere | |||||
| Inferior frontal gyrus/insula | 50 | 24 | -3 | 1607 | 8.00 |
| Middle frontal gyrus | 38 | 5 | 45 | 581 | 7.22 |
| Middle/superior temporal gyrus | 48 | -18 | -14 | 658 | 6.96 |
| Insula | 26 | 18 | -15 | 162 | 6.44 |
| Superior temporal gyrus | 44 | -35 | 7 | 143 | 6.07 |
| Angular gyrus | 42 | -63 | 26 | 93 | 5.92 |
| Inferior frontal gyrus | 54 | 29 | 18 | 146 | 5.60 |
| Inferior frontal gyrus | 38 | 15 | 32 | 50 | 5.27 |
| Cuneus | 21 | -74 | 12 | 31 | 5.13 |
| Middle frontal gyrus | 32 | 5 | 59 | 29 | 5.02 |
| Fusiform gyrus | 32 | -41 | -21 | 4 | 4.98 |
| Superior temporal gyrus | 65 | -4 | -6 | 4 | 4.97 |
| Inferior frontal gyrus | 41 | 24 | 15 | 4 | 4.83 |
| Inferior frontal gyrus | 48 | 38 | 11 | 1 | 4.77 |
Note: Fixed effext analyses, FWE-corrected, p < 0.05. Coordinates are given of the peak voxel in activated clusters.
In sum, whereas fMRI in healthy participants revealed activations in classical language areas including left inferior/middle frontal and left middle temporal regions, patients with stroke exhibited increased activations in right hemisphere homologues.
3.3.2. Group differences of language functioning and language laterality
As a group, the children with stroke exhibited significantly poorer language functions in all domains except writing compared to the healthy control group (Table 4). Furthermore, there was a significant difference between patient group and control group in language laterality, with the patients showing weaker left hemisphere involvement compared to the controls. However, within the stroke group, there was no significant difference between children with left and children with right hemisphere stroke in any language function or in language laterality.
Table 4. Group results and group differences in language functions and language laterality.
| Left stroke group |
Right stroke group |
Patients |
Controls |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | SD | Mean | Median | SD | U | p | Mean | Median | SD | Mean | Median | SD | U | p | ||||
| Language functioning | |||||||||||||||||||
| Naming | 0.00 | 0.71 | 1.43 | -0.48 | -0.52 | 1.56 | 32 | 0.673 | -0.23 | .31 | 1.46 | 0.89 | 1.13 | 0.68 | 81 | 0.015 | |||
| Comprehension | -0.56 | -0.50 | 0.79 | -0.47 | -0.38 | 1.26 | 32 | 0.743 | -0.52 | -0.50 | 1.00 | 0.44 | 0.59 | 0.76 | 69 | 0.005 | |||
| Reading | -0.69 | -0.40 | 1.00 | -0.42 | -0.23 | 1.35 | 29 | 0.541 | -0.57 | -0.40 | 1.15 | 0.43 | 0.58 | 0.64 | 69 | 0.005 | |||
| Writing | -0.53 | -1.02 | 1.02 | -0.09 | 0.67 | 1.30 | 26 | 0.321 | -0.32 | 0.33 | 1.15 | 0.18 | 0.67 | 0.94 | 103 | 0.096 | |||
| Word fluency | -.056 | -0.78 | 0.63 | -0.69 | -1.04 | 0.69 | 32 | 0.743 | -0.62 | -1.03 | 0.64 | 0.59 | 0.84 | 0.93 | 48 | 0.000 | |||
| Verbal memory | -0.75 | -1.01 | 0.98 | -0.27 | -0.27 | 0.83 | 27 | 0.423 | -0.52 | -0.47 | 0.92 | 0.37 | 0.50 | 1.03 | 77 | 0.011 | |||
| Language lateralization | |||||||||||||||||||
| LI | 0.30 | 0.55 | 0.13 | 0.39 | 0.64 | 36 | 0.963 | 0.14 | 0.30 | 0.57 | 0.59 | 0.71 | 0.35 | 72 | 0.006 | ||||
Note: Significance after Bonferroni correction is indicated with bold letters.
Abbreviation: LI = Language Laterality Index
3.3.3. Influence of demographic and clinical data on language functioning and language lateralization
Lesion size at stroke presentation (modASPECTS), residual lesion volume at study, age at stroke, age at testing, or time between stroke and testing were not significantly associated with any language function (Table 5). A standardized analysis of the lesion site effect on language performance and laterality was not possible due to the small sample size. However, we analyzed a possible influence of cortical or thalamic involvement in residual lesion and found that children with cortical involvement in the residual lesion (12/17) did not perform significantly worse in any language test (p >.082), nor did children with residual thalamic lesions (5/17; p > .279).
Table 5. Correlation of language functioning and language lateralization with demographic and clinical data, and difference by gender.
| Age at stroke | Time between stroke and study | modASPECTS at stroke presentation | Residual lesion volume at study | Age at study | Difference by gender | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Controls | Patients | Controls | ||||||||||||||||||
| r | p | r | p | r | p | r | p | r | p | r | p | U | p | U | p | ||||||
| Language functioning | |||||||||||||||||||||
| naming | 0.31 | 0.222 | -0.11 | 0.676 | 0.03 | 0.911 | -0.03 | 0.888 | 0.28 | 0.269 | 0.24 | 0.345 | 12 | 0.064 | 17 | 0.044 | |||||
| comprehension | 0.25 | 0.323 | 0.04 | 0.885 | -0.42 | 0.093 | -0.29 | 0.260 | 0.24 | 0.363 | 0.29 | 0.241 | 19 | 0.279 | 23 | 0.151 | |||||
| reading | 0.16 | 0.548 | 0.19 | 0.471 | -0.38 | 0.137 | 0.02 | 0.933 | 0.41 | 0.103 | -0.04 | 0.880 | 24 | 0.574 | 27 | 0.328 | |||||
| writing | 0.55 | 0.023 | 0.11 | 0.688 | -0.19 | 0.463 | -0.25 | 0.327 | 0.73 | 0.001 | 0.32 | 0.201 | 19 | 0.279 | 8 | 0.004 | |||||
| word fluency | 0.15 | 0.558 | 0.04 | 0.869 | -0.14 | 0.581 | -0.16 | 0.533 | 0.36 | 0.162 | -0.09 | 0.732 | 10 | 0.037 | 25 | 0.246 | |||||
| verbal memory | 0.26 | 0.319 | -0.09 | 0.722 | -0.38 | 0.137 | -0.26 | 0.314 | 0.22 | 0.395 | 0.29 | 0.239 | 16 | 0.160 | 22 | 0.151 | |||||
| Language lateralization | |||||||||||||||||||||
| LI | 0.23 | 0.371 | -0.12 | 0.653 | 0.17 | 0.505 | 0.00 | 0.996 | 0.28 | 0.275 | -0.36 | 0.143 | 16 | 0.130 | 38 | 0.930 | |||||
Note: Significance after Bonferroni correction is indicated with bold letters.
Abbreviation: LI = Language Laterality Index; modASPECTS = Alberta Stroke Program Early Computed Tomography Score
There was a significant positive correlation between age at examination and writing in the stroke group (rho = 0.73, p = 0.001), but not in the control group. Vice versa, a gender effect was observed in writing performance in the healthy controls, where girls were significantly better than boys (U(n1 = 7, n2 = 11) = 8, p = 0.004, r = 0.68), but not in the stroke group. When calculated for the whole group of study participants, age at examination did not correlate with any language test, but the gender effect was larger in girls outperforming boys in naming (U(n1 = 12, n2 = 23) = 61, p = 0.006, r = 0.46) and writing (U(n1 = 12, n2 = 23) =55, p = 0.003, r = 0.51).
Moreover, language laterality did not correlate with any demographic data, and remarkably neither correlated with lesion volume at stroke presentation, cortical or thalamic involvement in residual lesion, nor residual lesion volume at study.
3.3.4. The association of language functioning and language lateralization
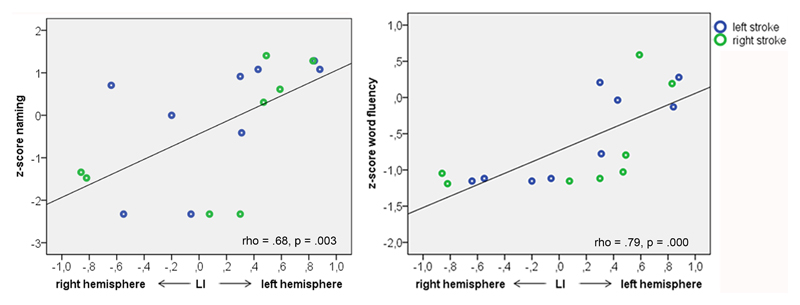
In stroke patients, nonparametric correlation analysis revealed a significant relationship between language laterality and naming (rho = 0.68, p = 0.003) and word fluency (rho = 0.79, p < 0.001), respectively (Figure 4, Table 6). This indicates a positive effect of left-lateralized language function on naming and word fluency.
Figure 4.
Association of language laterality and language functioning. The association of LI and naming and word fluency respectively, in stroke patients.
Table 6. Correlation of language laterality with language functioning.
| Stroke patients | Healthy controls | ||||
|---|---|---|---|---|---|
| rho | p | rho | p | ||
| Naming | 0.68 | 0.003 | -0.44 | 0 .065 | |
| Comprehension | 0.04 | 0.892 | -0.35 | 0 .150 | |
| Reading | 0.12 | 0.163 | -0.48 | 0 .044 | |
| Writing | 0.35 | 0.163 | 0.17 | 0 .497 | |
| Word fluency | 0.79 | 0.000 | -0.11 | 0.659 | |
| Verbal memory | 0.20 | 0.442 | -0.14 | 0.589 | |
Note: Significance after Bonferroni correction is indicated with bold letters.
Abbreviation: LI = Language Laterality Index
There was no association between LI and comprehension, reading, writing, or verbal memory in stroke patients. In healthy controls, there was a trend for less left language lateralization in children with better naming and reading, but correlations did non survive Bonferroni correction.
In summary, typical language laterality was associated with better naming and word fluency, whereas atypical (i.e. bilateral or right-lateralized) language representation was unfavorable for language outcome in children with stroke. In healthy controls, no significant association of language lateralization and functioning was found.
4. Discussion
The present study investigated language abilities and language localization in 17 children with unilateral childhood stroke and 18 healthy controls. Language deficits and increased involvement of right hemisphere homologues of typical language areas were found in both children with left- and children with right-sided stroke. Language abilities were not dependent on lesion size at stroke presentation, lesion volume at study, lesion side, lesion site, or age at stroke, but were significantly associated with language representation: typical language laterality was associated with better naming and word fluency, whereas atypical language laterality was unfavorable for language outcome in children with stroke.
4.1. The relationship between language lateralization and language performance in neuropediatric populations
Our findings of better language abilities in left language lateralization following childhood stroke are in line with the study of Elkana et al. which found increased language proficiency associated with stronger lateralization to the left hemisphere in seven children with left-sided stroke acquired during childhood. 14 This association has also been reported in children with left perinatal stroke, where better expressive and receptive language abilities were associated with increased activations in left hemispheric language regions. 12 Support comes also from studies in other neuropediatric populations: Lillywhite et al. investigated children with benign childhood epilepsy with centrotemporal spikes and found better sentence production correlated with increasing left-sided language lateralization. 39 Furthermore, de Guibert et al. described significantly less left lateralization in children with developmental dysphasia compared to healthy controls. 40 These results together with the findings of the present study indicate that functional language organization favouring the left hemisphere in children is associated with better language outcome. In contrast, Lidzba et al. investigated twelve patients, five of whom where children, who suffered from a left hemisphere stroke in childhood and found right lateralized language to be favorable for language outcome. 15 However, language outcome in this study was only examined by a questionnaire to the patient or the parents, which limits information on language abilities. The present study shows that the relationship between language functioning and language lateralization is present across a wide range of lesion volumes and irrespective of the hemispheric side of the lesion. This critical role of the left hemisphere even in left hemisphere stroke supports the view of an ontogenetic predisposition of the left hemisphere for the maturation of linguistic functions. 12 Children with left hemisphere language regions spared by or recovered after stroke may develop normal or near-to-normal language functions, whereas children with language regions affected by stroke may develop bilateral or right language lateralization which represents a substitutive mechanism. 41 This substitution of language areas seems to be associated with reduced efficiency.
4.2. The relationship between language lateralization and language performance in healthy children
In the healthy control group, we did not observe an association of language laterality and language functioning. However, this finding should be interpreted with caution, as the variability of language lateralization was limited in this group: only two children showed atypical language lateralization. Whereas a distribution of 11 % of healthy children with atypical language lateralization corresponds to previous findings of studies with larger populations, 42 the group of healthy children with atypical language lateralization observed in this study is not large enough to perform reliable statistical analyses. Thus, a possible relationship may become recognizable with a larger study population of healthy children with a more distributed LI. Previous literature in healthy children and adolescents found an association between language lateralization and functioning, but conflicting findings were reported regarding the nature of this relationship: some studies found a positive correlation between language lateralization towards the left hemisphere and verbal abilities, 43, 44 whereas others reported better language performance related to less lateralization and more involvement of the contralateral hemisphere. 29, 30, 45–47 The contradictory findings of these studies may be the result of different imaging methods and different tasks testing different functions. The relationship between lateralization and cognitive functioning has been reported as dependent on task demands. 48, 49 Furthermore, the recruitment of right homologues may reflect a different underlying mechanism in healthy children compared to children with disorders.
4.3. Language reorganization following left- or right-hemispheric childhood stroke
Right lateralized language following left hemisphere stroke has already been reported in studies on perinatal stroke 12, 50–52 and in studies on childhood stroke. 9, 13–15, 41, 53, 54 These studies have reported increased activity in right hemisphere areas homotopic to left hemisphere language regions after childhood stroke. Recent findings of Westmacott et al. suggest that reorganization of language function may be more likely to involve bilateral recruitment following left stroke in childhood than in adulthood. 54 It is therefore suggested that in children with left hemisphere stroke, the right hemisphere takes over language functions that are typically proceeded in the left hemisphere. 55, 56 The present study confirms these findings: While fMRI in healthy participants revealed activations in classical language areas including left inferior/middle frontal and left middle temporal regions, patients with stroke exhibited increased activations in right hemisphere homologues. We found atypical (i.e., bilateral or right) language lateralization in 50 % of children with left hemisphere stroke, whereas only 11 % of healthy controls showed atypical language lateralization. However, we furthermore included children with right hemisphere stroke in our study; interestingly, atypical language lateralization was also present in this group. In sum, 33 % of them (3/9) showed bilateral or right lateralized language despite of a lesion in the right hemisphere, and despite right-handedness. On the group level, not only language abilities, but also the LI did not differ significantly between left- and right-sided stroke. This supports the findings of Everts et al. who investigated five children with right stroke and found two of them with atypical language dominance. 57 Both their study and ours are limited due to their small sample size; thus, it may only be by chance that three of our right-sided stroke patients, and two in the Evert’s study, showed atypical language representation due to the well-known interindividual variability of language dominance. However, to the best of our knowledge, no other pediatric study investigated language localization following right hemisphere stroke on a group level. Thus, at the moment, we may only hypothesize that the complex interplay between the two hemispheres is disturbed not only after left, but also after right hemisphere stroke, resulting in an increased involvement of right-sided brain areas that are part of the intrinsic language network. The increased right language lateralization following both left- and right stroke could further be explained by nonspecific mechanisms in language tasks: additional attentional processes may enhance right hemisphere involvement in language in patients with brain lesions. 58 This hypothesis is, in fact, supported by studies underpinning the role of the right hemisphere in auditory selective attention. 59–61 Furthermore, recovery studies in adults suffering from aphasia after left hemisphere stroke demonstrate that right hemisphere recruitment often peaks early in recovery and decreases over time in association with clinical improvements. 62–65 These findings together with the present results indicate that right hemisphere regions could be engaged in an attentional component specific to language processing.
4.4. Cognitive outcome after childhood stroke
Most studies on the cognitive outcome after childhood stroke reported reduced abilities predominantly in executive functions, processing speed, and working memory, whereas verbal abilities, most often measured as verbal IQ, were much better preserved and often described as normal. 66–68 However, the verbal IQ is a gross measure incorporating also non-linguistic tests like arithmetics and digit span, which may blur the language findings. When language functions are investigated with linguistic measurements in detail, many children with stroke do show subtle to profound language deficits. 6–9, 69 Our present study corroborates these findings: We administered an extensive language test battery in our study population with the aim to obtain a detailed language profile of every child. We found below average language functions in 71 % of the stroke study group, with 30 % of them even having impaired language abilities in one or more linguistic domains. Considering that months to years have passed since stroke and children had time to recover, many of them still have to face persistent language problems. It is well known that language problems in childhood are associated with poor academic outcomes. These post-stroke language deficits will thus influence their professional development and psychosocial aspects of their life.
Whereas some studies reported language deficits both after left and right-sided stroke without a significant difference between groups, 7, 9, 70 others could locate post stroke deficits in language production and language comprehension to the hemispheric side of the lesion and showed that children with lesions in the left temporal lobe had more profound impairment in word production, whereas right-sided stroke caused more impairment in comprehension and gesture. 71–73 In the present study, we did not observe a lesion-side specific pattern: the groups of children with left- versus right-sided stroke did not significantly differ in any language domain. A closer look at the individual linguistic profiles revealed impaired and below-average language functions in both groups, with naming and written language capabilities being most often reduced.
4.5. The effect of lesion site on language performance
Though language impairment has classically been labelled as a cortical deficit, studies in adult stroke often report language deficits following subcortical stroke. Especially thalamic and basal ganglia lesions have been shown to result in aphasic symptoms, yet, the specific functions of these subcortical structures remain rather controversial. 74, 75 The present study investigated the effect of cortical/subcortical and thalamic involvement, respectively, on language functioning and did not find any effect of lesion location on language performance. However, statistical analyses are difficult due to the small sample size and the large heterogeneity in lesion site and lesion volume in this study. Predicting an individual's language outcome after stroke on the basis of lesion site has been shown to be already difficult in adult stroke despite the use of large databases, e.g. PLORAS; 76 it is yet impossible to date in studies with small numbers of children who suffered from stroke.
4.6. The effect of age at stroke on cognitive outcome
Previous literature has shown effects of age at stroke on cognitive outcome. However, studies investigating this question often compared perinatal and neonatal stroke outcome with childhood stroke outcome, and their findings were contradictory, with some studies revealing better cognitive outcome in perinatal stroke compared to childhood stroke, 13 and others reporting worse outcome in stroke aquired during the perinatal phase compared to later stroke. 7, 9, 77, 78 Yet, perinatal stroke and neonatal stroke may have different underlying pathologies compared to childhood stroke, which may potentially influence outcome. 79 Furthermore, perinatal and neonatal strokes have a higher risk of post-stroke epilepsy and other co-morbidities, which is well known to have a negative impact on cognition. 80 Studies investigating a possible age effect on language outcome in childhood stroke alone did no find an association between age at stroke and language functioning. 41, 81, 82 This is in line with our study which reveals no significant association between age at stroke and language performance. Yet, given the rarity of childhood stroke, sample sizes were often small, as in the present study. Thus, the effect of age at stroke may be difficult to statistically disentangle from other associated variables. However, such an effect might not exist at all, as a recent study on long-term outcome following ischemic stroke, including 95 children and 154 young adults, did also not find a significant difference between functional outcome of childhood and young adult stroke. 83
4.7. Limitations
Some limitations of our study have to be considered. First, the patient group is rather small, which limits the interpretability of results. The small sample size is due to the rarity of disease, and in addition a consequence of our strict inclusion criteria: unilateral stroke in previously healthy children and the absence of epilepsy at examination further restricts the inclusion of possible participants. Second, both age at examination and age at stroke show large variability. Statistical analyses do not show a significant influence of these demographic factors on outcome or language laterality. Nonetheless, they may influence outcome parameters. To overcome this limitation, individual language profiles and interpretation of intrasubject correlations are important apects of this study. Third, the period between stroke and examination also varies. Whereas some children were tested years post stroke, others experienced their stroke only several months before examination. Thus, reorganizational processes may not be completed in the latter. Though no correlation between post-stroke period and neither outcome nor language laterality was found, it may probably have an influence on individual results and has to be taken into account when interpreting the results of the study. Whereas the findings of a relationship between language laterality and language functioning remain unaffected by the post-stroke period, conclusions about long-term language outcome of childhood stroke may be limited. Fourth, recording of in-scanner task accuracy was missing due to technical reasons in many participants. While on-site check of in-scanner performance showed adequate response during the fMRI paradigm in all participants, a quantitative analysis of task performance and the investigation of the relationship between in-scanner performance, out-scanner performance, and language laterality probably may provide important additional information.
4.8. Conclusions
Children with focal brain lesions frequently demonstrate atypical language representations with increased involvement of right hemispheric language areas. However, these alterations of functional language representation seem to be insufficient in compensating for deficits in various language domains. Right homologues do not have the same cognitive capacity, even in young children. Atypical right hemispheric language representation after childhood stroke may represent the functionally insufficient compensation for language network alterations, rather than true brain “plasticity” or “reorganization” – terms which are frequently cited in this context.
Supplementary Material
Individual language profiles in healthy controls. The solid line represents SD -2 (impaired function), the dashed line SD -1 (below average function).
Group activations (one sample t-test, pFWE < .05) of the healthy control group on the 3D rendered brain of one participant. Left is left-hemisphere (LH).
Acknowledgements
The authors would like to thank the members of the Pediatric Stroke Board of the Medical University Vienna (Francesco Cardona, Christoph Male, Marie-Therese Schmook, and Katharina Thom) for their clinical support.
Funding
This work was supported by the Austrian Science Fund (FWF), Grant KLI544-B27 and by the Oesterreichische Nationalbank (Anniversary Fund, project number 15356).
Abbreviations
- EHI
Edinburgh Handedness Inventory
- fMRI
functional MRI
- LI
language laterality index
- modASPECTS
Alberta Stroke Program Early Computed Tomography Score
- SD
standard deviation
Footnotes
Conflicts of interest
The authors have no conflicts of interests.
References
- 1.George MG, Tong X, Kuklina EV, Labarthe DR. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995-2008. Ann Neurol. 2011;70(5):713–21. doi: 10.1002/ana.22539. [DOI] [PubMed] [Google Scholar]
- 2.Lynch JK, Hirtz DG, Deveber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109(1):116–23. doi: 10.1542/peds.109.1.116. [DOI] [PubMed] [Google Scholar]
- 3.Steinlin M. A clinical approach to arterial ischemic childhood stroke: increasing knowledge over the last decade. Neuropediatrics. 2012;43(1):1–9. doi: 10.1055/s-0032-1307449. [DOI] [PubMed] [Google Scholar]
- 4.Cardenas JF, Rho JM, Kirton A. Pediatric stroke. Childs NervSyst. 2011;27(9):1375–90. doi: 10.1007/s00381-010-1366-9. [DOI] [PubMed] [Google Scholar]
- 5.Ballantyne AO, Spilkin AM, Hesselink J, Trauner DA. Plasticity in the developing brain: intellectual, language and academic functions in children with ischaemic perinatal stroke. Brain. 2008;131(Pt 11):2975–85. doi: 10.1093/brain/awn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman HM, MacWhinney B, Sacco K. Sentence processing in children with early unilateral brain injury. Brain Lang. 2002;83(2):335–52. doi: 10.1016/s0093-934x(02)00037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman SB, Max JE, Gamino JF, McGlothlin JH, Cliff SN. Discourse plasticity in children after stroke: age at injury and lesion effects. Pediatr Neurol. 2003;29(1):34–41. doi: 10.1016/s0887-8994(03)00012-2. [DOI] [PubMed] [Google Scholar]
- 8.Pitchford NJ. Spoken language correlates of reading impairments acquired in childhood. Brain Lang. 2000;72(2):129–49. doi: 10.1006/brln.1999.2267. [DOI] [PubMed] [Google Scholar]
- 9.Avila L, Riesgo R, Pedroso F, Goldani M, Danesi M, Ranzan J, et al. Language and focal brain lesion in childhood. J Child Neurol. 2010;25(7):829–33. doi: 10.1177/0883073809350724. [DOI] [PubMed] [Google Scholar]
- 10.Westmacott R, MacGregor D, Askalan R, deVeber G. Late emergence of cognitive deficits after unilateral neonatal stroke. Stroke. 2009;40(6):2012–9. doi: 10.1161/STROKEAHA.108.533976. [DOI] [PubMed] [Google Scholar]
- 11.Anderson V, Spencer-Smith M, Wood A. Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain. 2011;134(Pt 8):2197–221. doi: 10.1093/brain/awr103. [DOI] [PubMed] [Google Scholar]
- 12.Raja Beharelle A, Dick AS, Josse G, Solodkin A, Huttenlocher PR, Levine SC, et al. Left hemisphere regions are critical for language in the face of early left focal brain injury. Brain. 2010;133(Pt 6):1707–16. doi: 10.1093/brain/awq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilves P, Tomberg T, Kepler J, Laugesaar R, Kaldoja ML, Kepler K, et al. Different plasticity patterns of language function in children with perinatal and childhood stroke. J Child Neurol. 2014;29(6):756–64. doi: 10.1177/0883073813489350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkana O, Frost R, Kramer U, Ben-Bashat D, Hendler T, Schmidt D, et al. Cerebral reorganization as a function of linguistic recovery in children: An fMRI study. Cortex. 2011;47(2):202–16. doi: 10.1016/j.cortex.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Lidzba K, Kupper H, Kluger G, Staudt M. The time window for successful right-hemispheric language reorganization in children. Eur J Paediatr Neurol. 2017;21(5):715–21. doi: 10.1016/j.ejpn.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 16.McGhee RL, Ehrler DJ, DiSimoni F. TTFC-2. The Token Test for Children. Austin, TX: ProEd; 2007. [Google Scholar]
- 17.Deák GO. Interrelations of language and cognitive development. In: Brooks PJ, Kempe V, editors. Encyclopedia of Language Development. Thousend Oaks, CA: Edition SAGE; 2014. pp. 284–91. [Google Scholar]
- 18.Glück CW. Wortschatz- und Wortfindungstest für 6- bis 10-Jährige. München: Elsevier; 2011. [Google Scholar]
- 19.Fox AV. TROG-D - Test zur Überprüfung des Grammatik-Verständnisses. Idstein: Schulz-Kirschner; 2008. [Google Scholar]
- 20.Moll K, Landerl K. Lese- und Rechtschreibtest SLRT II. Bern: Huber; 2010. [Google Scholar]
- 21.Grissemann H, Lindner M. Zürcher Lesetest: ZLT, Förderdiagnostik bei gestörtem Schrifterwerb. Bern: Verlag Hans Huber; 2000. [Google Scholar]
- 22.Aschenbrenner S, Tucha O, Lange KW. Regensburger Wortflüssigkeitstest. Göttingen: Testzentrale; 2001. [Google Scholar]
- 23.Lezak MD. Neuropsychological assessment. New York: Oxford university Press; 1995. [Google Scholar]
- 24.Helmstädter C, Lendt M, Lux S. Verbaler Lern- und Merkfähigkeitstest VLMT. Göttingen: Beltz Test; 2001. [Google Scholar]
- 25.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 26.Berl MM, Mayo J, Parks EN, Rosenberger LR, VanMeter J, Ratner NB, et al. Regional differences in the developmental trajectory of lateralization of the language network. Hum Brain Mapp. 2014;35(1):270–84. doi: 10.1002/hbm.22179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sepeta LN, Berl MM, Wilke M, You X, Mehta M, Xu B, et al. Age-dependent mesial temporal lobe lateralization in language fMRI. Epilepsia. 2016;57(1):122–30. doi: 10.1111/epi.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelletier I, Paquette N, Lepore F, Rouleau I, Sauerwein CH, Rosa C, et al. Language lateralization in individuals with callosal agenesis: an fMRI study. Neuropsychologia. 2011;49(7):1987–95. doi: 10.1016/j.neuropsychologia.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 29.Bartha-Doering L, Kollndorfer K, Kasprian G, Novak A, Schuler AL, Fischmeister FP, et al. Weaker Semantic Language Lateralization Associated with Better Semantic Language Performance in Healthy Right-handed Children. Brain Behav. 2018 doi: 10.1002/brb3.1072. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartha-Doering L, Novak A, Kollndorfer K, Kasprian G, Schuler AL, Berl MM, et al. When two are better than one: Bilateral mesial temporal lobe contributions associated with better vocabulary skills in children and adolescents. Brain Lang. 2018;184:1–10. doi: 10.1016/j.bandl.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norrelgen F, Lilja A, Ingvar M, Amark P, Fransson P. Presurgical language lateralization assessment by fMRI and dichotic listening of pediatric patients with intractable epilepsy. NeuroImage Clinical. 2015;7:230–9. doi: 10.1016/j.nicl.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmad Z, Balsamo LM, Sachs BC, Xu B, Gaillard WD. Auditory comprehension of language in young children: neural networks identified with fMRI. Neurology. 2003;60(10):1598–605. doi: 10.1212/01.wnl.0000059865.32155.86. [DOI] [PubMed] [Google Scholar]
- 33.Röder B, Stock O, Neville H, Bien S, Rosler F. Brain activation modulated by the comprehension of normal and pseudo-word sentences of different processing demands: a functional magnetic resonance imaging study. Neuroimage. 2002;15(4):1003–14. doi: 10.1006/nimg.2001.1026. [DOI] [PubMed] [Google Scholar]
- 34.Andersen SM, Rapcsak SZ, Beeson PM. Cost function masking during normalization of brains with focal lesions: still a necessity? Neuroimage. 2010;53(1):78–84. doi: 10.1016/j.neuroimage.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage. 2001;14(2):486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- 36.Wilke M, Holland SK, Altaye M, Gaser C. Template-O-Matic: a toolbox for creating customized pediatric templates. Neuroimage. 2008;41(3):903–13. doi: 10.1016/j.neuroimage.2008.02.056. [DOI] [PubMed] [Google Scholar]
- 37.Wilke M, Lidzba K. LI-tool: a new toolbox to assess lateralization in functional MR-data. J Neurosci Methods. 2007;163(1):128–36. doi: 10.1016/j.jneumeth.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 38.Beslow LA, Vossough A, Dahmoush HM, Kessler SK, Stainman R, Favilla CG, et al. Modified Pediatric ASPECTS Correlates with Infarct Volume in Childhood Arterial Ischemic Stroke. Front Neurol. 2012;3:122. doi: 10.3389/fneur.2012.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lillywhite LM, Saling MM, Harvey AS, Abbott DF, Archer JS, Vears DF, et al. Neuropsychological and functional MRI studies provide converging evidence of anterior language dysfunction in BECTS. Epilepsia. 2009;50(10):2276–84. doi: 10.1111/j.1528-1167.2009.02065.x. [DOI] [PubMed] [Google Scholar]
- 40.de Guibert C, Maumet C, Jannin P, Ferre JC, Treguier C, Barillot C, et al. Abnormal functional lateralization and activity of language brain areas in typical specific language impairment (developmental dysphasia) Brain. 2011;134(Pt 10):3044–58. doi: 10.1093/brain/awr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chilosi AM, Cipriani P, Pecini C, Brizzolara D, Biagi L, Montanaro D, et al. Acquired focal brain lesions in childhood: effects on development and reorganization of language. Brain Lang. 2008;106(3):211–25. doi: 10.1016/j.bandl.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Szaflarski JP, Rajagopal A, Altaye M, Byars AW, Jacola L, Schmithorst VJ, et al. Left-handedness and language lateralization in children. Brain Res. 2012;1433:85–97. doi: 10.1016/j.brainres.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Everts R, Lidzba K, Wilke M, Kiefer C, Mordasini M, Schroth G, et al. Strengthening of laterality of verbal and visuospatial functions during childhood and adolescence. Hum Brain Mapp. 2009;30(2):473–83. doi: 10.1002/hbm.20523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berl MM, Duke ES, Mayo J, Rosenberger LR, Moore EN, VanMeter J, et al. Functional anatomy of listening and reading comprehension during development. Brain Lang. 2010;114(2):115–25. doi: 10.1016/j.bandl.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeatman JD, Ben-Shachar M, Glover GH, Feldman HM. Individual differences in auditory sentence comprehension in children: An exploratory event-related functional magnetic resonance imaging investigation. Brain Lang. 2010;114(2):72–9. doi: 10.1016/j.bandl.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lidzba K, Schwilling E, Grodd W, Krageloh-Mann I, Wilke M. Language comprehension vs. language production: age effects on fMRI activation. Brain Lang. 2011;119(1):6–15. doi: 10.1016/j.bandl.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Booth JR, MacWhinney B, Thulborn KR, Sacco K, Voyvodic JT, Feldman HM. Developmental and lesion effects in brain activation during sentence comprehension and mental rotation. Dev Neuropsychol. 2000;18(2):139–69. doi: 10.1207/S15326942DN1802_1. [DOI] [PubMed] [Google Scholar]
- 48.Boles DB, Barth JM, Merrill EC. Asymmetry and performance: toward a neurodevelopmental theory. Brain Cogn. 2008;66(2):124–39. doi: 10.1016/j.bandc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Piervincenzi C, Petrilli A, Marini A, Caulo M, Committeri G, Sestieri C. Multimodal assessment of hemispheric lateralization for language and its relevance for behavior. Neuroimage. 2016;142:351–70. doi: 10.1016/j.neuroimage.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 50.Lidzba K, Wilke M, Staudt M, Krageloh-Mann I, Grodd W. Reorganization of the cerebro-cerebellar network of language production in patients with congenital left-hemispheric brain lesions. Brain Lang. 2008;106(3):204–10. doi: 10.1016/j.bandl.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Szaflarski JP, Allendorfer JB, Byars AW, Vannest J, Dietz A, Hernando KA, et al. Age at stroke determines post-stroke language lateralization. Restor Neurol Neurosci. 2014;32(6):733–42. doi: 10.3233/RNN-140402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tillema JM, Byars AW, Jacola LM, Schapiro MB, Schmithorst VJ, Szaflarski JP, et al. Cortical reorganization of language functioning following perinatal left MCA stroke. Brain Lang. 2008;105(2):99–111. doi: 10.1016/j.bandl.2007.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elkana O, Frost R, Kramer U, Ben-Bashat D, Schweiger A. Cerebral language reorganization in the chronic stage of recovery: a longitudinal fMRI study. Cortex. 2013;49(1):71–81. doi: 10.1016/j.cortex.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Westmacott R, McAndrews MP, deVeber G. Language Representation Following Left MCA Stroke in Children and Adults: An fMRI Study. Can J Neurol Sci. 2017;44(5):483–97. doi: 10.1017/cjn.2017.44. [DOI] [PubMed] [Google Scholar]
- 55.Staudt M, Grodd W, Niemann G, Wildgruber D, Erb M, Krageloh-Mann I. Early left periventricular brain lesions induce right hemispheric organization of speech. Neurology. 2001;57(1):122–5. doi: 10.1212/wnl.57.1.122. [DOI] [PubMed] [Google Scholar]
- 56.Lidzba K, Staudt M. Development and (re)organization of language after early brain lesions: capacities and limitation of early brain plasticity. Brain Lang. 2008;106(3):165–6. doi: 10.1016/j.bandl.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Everts R, Lidzba K, Wilke M, Kiefer C, Wingeier K, Schroth G, et al. Lateralization of cognitive functions after stroke in childhood. Brain Inj. 2010;24(6):859–70. doi: 10.3109/02699051003724978. [DOI] [PubMed] [Google Scholar]
- 58.Papathanassiou D, Etard O, Mellet E, Zago L, Mazoyer B, Tzourio-Mazoyer N. A common language network for comprehension and production: a contribution to the definition of language epicenters with PET. Neuroimage. 2000;11(4):347–57. doi: 10.1006/nimg.2000.0546. [DOI] [PubMed] [Google Scholar]
- 59.Heilman KM, Van Den Abell T. Right hemisphere dominance for attention: the mechanism underlying hemispheric asymmetries of inattention (neglect) Neurology. 1980;30(3):327–30. doi: 10.1212/wnl.30.3.327. [DOI] [PubMed] [Google Scholar]
- 60.Somers DC, Sheremata SL. Attention maps in the brain. Wiley Interdiscip Rev Cogn Sci. 2013;4(4):327–40. doi: 10.1002/wcs.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tzourio N, Massioui FE, Crivello F, Joliot M, Renault B, Mazoyer B. Functional anatomy of human auditory attention studied with PET. Neuroimage. 1997;5(1):63–77. doi: 10.1006/nimg.1996.0252. [DOI] [PubMed] [Google Scholar]
- 62.Fernandez B, Cardebat D, Demonet JF, Joseph PA, Mazaux JM, Barat M, et al. Functional MRI follow-up study of language processes in healthy subjects and during recovery in a case of aphasia. Stroke. 2004;35(9):2171–6. doi: 10.1161/01.STR.0000139323.76769.b0. [DOI] [PubMed] [Google Scholar]
- 63.Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, et al. Dynamics of language reorganization after stroke. Brain. 2006;129(Pt 6):1371–84. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- 64.Kurland J, Cortes CR, Wilke M, Sperling AJ, Lott SN, Tagamets MA, et al. Neural Mechanisms Underlying Learning following Semantic Mediation Treatment in a case of Phonologic Alexia. Brain Imaging Behav. 2008;2(3):147. doi: 10.1007/s11682-008-9027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Breier JI, Randle S, Maher LM, Papanicolaou AC. Changes in maps of language activity activation following melodic intonation therapy using magnetoencephalography: two case studies. J Clin Exp Neuropsychol. 2010;32(3):309–14. doi: 10.1080/13803390903029293. [DOI] [PubMed] [Google Scholar]
- 66.Studer M, Boltshauser E, Capone Mori A, Datta A, Fluss J, Mercati D, et al. Factors affecting cognitive outcome in early pediatric stroke. Neurology. 2014;82(9):784–92. doi: 10.1212/WNL.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 67.Westmacott R, Askalan R, MacGregor D, Anderson P, Deveber G. Cognitive outcome following unilateral arterial ischaemic stroke in childhood: effects of age at stroke and lesion location. Dev Med Child Neurol. 2010;52(4):386–93. doi: 10.1111/j.1469-8749.2009.03403.x. [DOI] [PubMed] [Google Scholar]
- 68.O'Keeffe F, Liegeois F, Eve M, Ganesan V, King J, Murphy T. Neuropsychological and neurobehavioral outcome following childhood arterial ischemic stroke: attention deficits, emotional dysregulation, and executive dysfunction. Child Neuropsychol. 2014;20(5):557–82. doi: 10.1080/09297049.2013.832740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reilly JS, Bates EA, Marchman VA. Narrative discourse in children with early focal brain injury. Brain Lang. 1998;61(3):335–75. doi: 10.1006/brln.1997.1882. [DOI] [PubMed] [Google Scholar]
- 70.Max JE. Effect of side of lesion on neuropsychological performance in childhood stroke. J Int Neuropsychol Soc. 2004;10(5):698–708. doi: 10.1017/S1355617704105092. [DOI] [PubMed] [Google Scholar]
- 71.Stiles J, Reilly J, Paul B, Moses P. Cognitive development following early brain injury: evidence for neural adaptation. Trends Cogn Sci. 2005;9(3):136–43. doi: 10.1016/j.tics.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 72.Bates E, Thal D, Trauner D, Fenson J, Aram D, Eisele J, et al. From first words to grammar in children with focal brain injury. Dev Neuropsychol. 1997;13(3):275–343. [Google Scholar]
- 73.Thal DJ, Marchman V, Stiles J, Aram D, Trauner D, Nass R, et al. Early lexical development in children with focal brain injury. Brain Lang. 1991;40(4):491–527. doi: 10.1016/0093-934x(91)90145-q. [DOI] [PubMed] [Google Scholar]
- 74.Sebastian R, Schein MG, Davis C, Gomez Y, Newhart M, Oishi K, et al. Aphasia or Neglect after Thalamic Stroke: The Various Ways They may be Related to Cortical Hypoperfusion. Front Neurol. 2014;5:231. doi: 10.3389/fneur.2014.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klostermann F, Krugel LK, Ehlen F. Functional roles of the thalamus for language capacities. Front Syst Neurosci. 2013;7:32. doi: 10.3389/fnsys.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Price CJ, Hope TM, Seghier ML. Ten problems and solutions when predicting individual outcome from lesion site after stroke. Neuroimage. 2017;145(Pt B):200–8. doi: 10.1016/j.neuroimage.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kolk A, Ennok M, Laugesaar R, Kaldoja ML, Talvik T. Long-term cognitive outcomes after pediatric stroke. Pediatr Neurol. 2011;44(2):101–9. doi: 10.1016/j.pediatrneurol.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 78.Max JE, Bruce M, Keatley E, Delis D. Pediatric stroke: plasticity, vulnerability, and age of lesion onset. J Neuropsychiatry Clin Neurosci. 2010;22(1):30–9. doi: 10.1176/jnp.2010.22.1.30. [DOI] [PubMed] [Google Scholar]
- 79.Grunt S, Mazenauer L, Buerki SE, Boltshauser E, Mori AC, Datta AN, et al. Incidence and outcomes of symptomatic neonatal arterial ischemic stroke. Pediatrics. 2015;135(5):e1220–8. doi: 10.1542/peds.2014-1520. [DOI] [PubMed] [Google Scholar]
- 80.Billinghurst LL, Beslow LA, Abend NS, Uohara M, Jastrzab L, Licht DJ, et al. Incidence and predictors of epilepsy after pediatric arterial ischemic stroke. Neurology. 2017;88(7):630–7. doi: 10.1212/WNL.0000000000003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chilosi AM, Pecini C, Cipriani P, Brizzolara D, Brovedani P, Ferretti G, et al. Cerebral language lateralization and early linguistic in children with focal brain lesions. In: Fabbro F, editor. Neurogenic language disorders in children. Oxford: Elsevier; 2004. pp. 49–63. [Google Scholar]
- 82.Martin IP. Persisten acquired childhood aphasia. In: Fabbro F, editor. Neurogenic language disorders in children. Oxford: Elsevier; 2004. pp. 231–51. [Google Scholar]
- 83.Goeggel Simonetti B, Cavelti A, Arnold M, Bigi S, Regenyi M, Mattle HP, et al. Long-term outcome after arterial ischemic stroke in children and young adults. Neurology. 2015;84(19):1941–7. doi: 10.1212/WNL.0000000000001555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Individual language profiles in healthy controls. The solid line represents SD -2 (impaired function), the dashed line SD -1 (below average function).
Group activations (one sample t-test, pFWE < .05) of the healthy control group on the 3D rendered brain of one participant. Left is left-hemisphere (LH).