Summary:
In the past decade, the resurgence of immunotherapy has changed the landscape of cancer therapy. Check point inhibitors targeting CTLA4 (Cytotoxic T-lymphocyte antigen-4), PD-1 (programmed death1) on lymphocytes and PD-L1 (programmed death ligand1) on tumors cells are currently utilized in the management of several cancers. These agents are double-edged sword with the positive effect being robust anti-tumor response but on the other side they can throttle up the normal immunologic homeostasis in a negative way, leading to adverse autoimmune toxicities. These adverse toxicities are frequent if patients have active auto-immune disorders. Here, we report a rare case of quiescent bullous pemphigoid which flared after initiation of pembrolizumab, a PD-L1 inhibitor.
Keywords: Immunotherapy, Pembrolizumab, Bullous Pemphigus, corticosteroids
Case Presentation:
A 64 year old male with a history of bullous pemphigoid under remission was diagnosed with bladder urothelial cancer. He initially presented with abdominal fullness, flank pain and labs revealed elevated creatinine of 8 mg/dl. CT scan of abdomen and pelvis revealed bilateral hydronephrosis with enlarged prostate and thickened bladder. On cystoscopy, he was noted to have a thickened, erythematous bladder and transurethral resection of bladder tumor (TURBT) was consistent with muscle invasive urothelial cancer. Due to the renal failure, he was not offered neoadjuvant chemotherapy and underwent radical cystectomy and pelvic lymph node dissection. The final pathology revealed bladder tumor extending into the extravesicular fat and multiple metastatic lymphadenopathy. He later received 6 cycles of cisplatin and gemcitabine. On surveillance, about 18 months later he was diagnosed with metastatic urothelial cancer. He received 6 cycles of carboplatin and gemcitabine following which he had progressive disease.
He was started on pembrolizumab due to disease progression. After the first treatment cycle, he developed a pruritic and painful skin rash on his face, trunk and extremities.
Investigations:
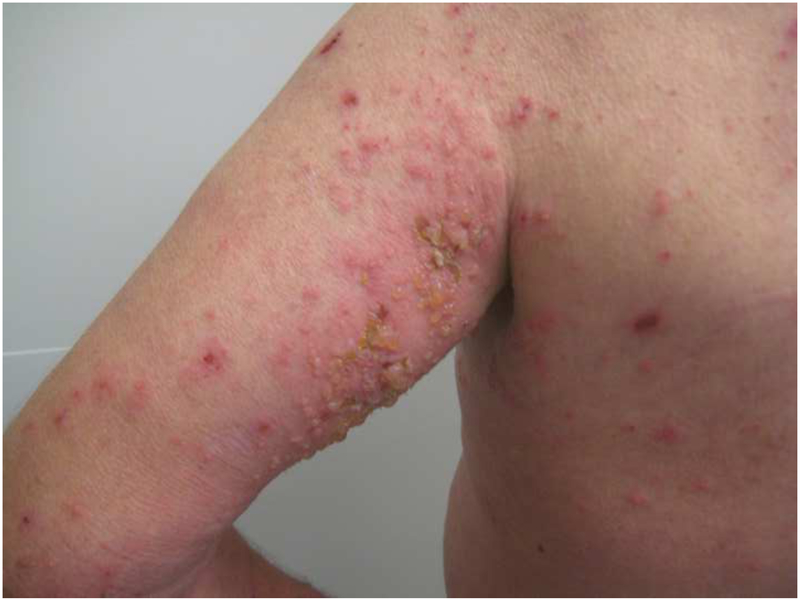
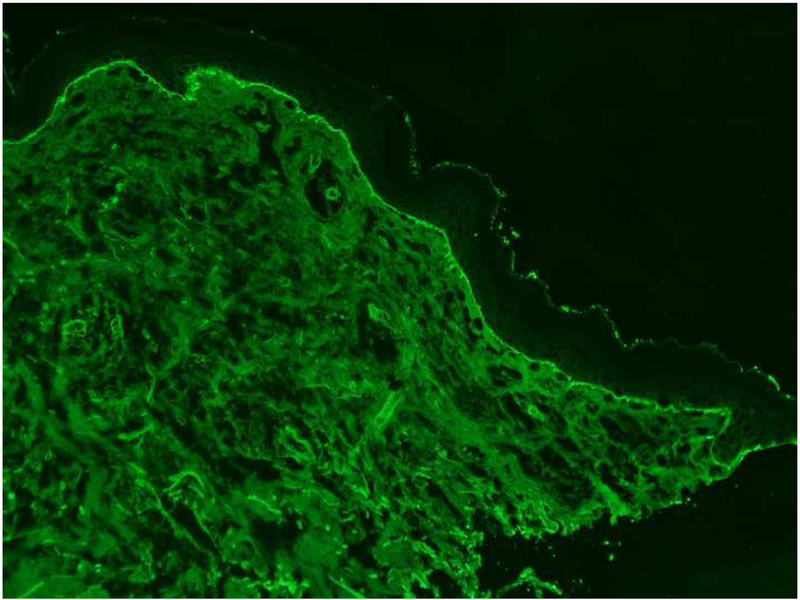
He was evaluated by Dermatology for the new-onset rash. On physical exam, he was noted to have scattered erosions with excoriations and numerous intact vesicles involving the scalp, cheeks, chest, bilateral arms and lower legs (Figure 1A, 1B). Shave biopsy of his right chest revealed subepidermal bullae with numerous eosinophils in the blister cavity and dermis, and perivascular inflammation consisting of eosinophils and lymphocytes. Direct immunofluorescence showed focal linear marking for IgG and diffuse linear marking for C3 along the dermal-epidermal junction. ELISA (enzyme-linked immunoassay) was positive (titer=69.22) for antibodies to bullous pemphigoid antigen 180 (BP180). These findings were consistent with his pre-existing diagnosis of bullous pemphigoid (Figure 2: H&E, Figure 3: DIF).
Figure 1A, 1B. Clinical images.
Numerous intact vesicles involving the trunk and extremities.
Figure 2. H&E.
H&E sections show subepidermal bullae with numerous eosinophils in the blister cavity and dermis, and perivascular inflammation consisting of eosinophils and lymphocytes.
Figure 3 A, B. DIF.
Direct immunofluorescence microscopy studies showing linear IgG (Figure 3A) and C3 (Figure 3B) along the dermal-epidermal junction.
Treatment:
Pembrolizumab was discontinued due to flaring of his bullous pemphigoid, and the patient was started on both systemic and topical corticosteroids. After several months of therapy, the skin lesions resolved. For the bladder cancer, he was started on paclitaxel and his bullous pemphigoid remained quiescent on low-dose prednisone and topical therapy.
Discussion:
In the past decade, the resurgence of immunotherapy has changed the landscape of cancer therapy. Programmed cell death PD-L1, a checkpoint inhibitor curtailing the host lymphocytic and apoptotic immune response, has been found to be overexpressed in malignant tumors2. Consequently, antibodies targeting PD-1 (found on T cells) or its receptor ligand PD-L1 have been shown to unbridle host antitumor processes, leading to in vivo tumor regression. Checkpoint inhibitors that inhibit cytotoxic T-lymphocyte antigen 4 (CTLA-4) and PD-1/PD-L1 are currently utilized in the treatment of several cancers1.
Platinum based chemotherapy was the only option for management of metastatic urothelial cancer until 2016, following which five checkpoint inhibitors (Nivolumab, pembrolizumab, Avelumab, Atezolizumab and Durvalumab) were approved for metastatic urothelial cancer.
However, checkpoint inhibitors are double-edged sword with the positive effect being robust anti-tumor response but on the other side, by blocking the negative regulators of immunity that are normally important for maintaining immunologic homeostasis, treatment can be associated with distinctive inflammatory adverse effects known as immune-related adverse events (irAEs)16. Multiple irAEs including hepatitis, colitis, pneumonitis, nephritis, endocrinopathies, and even reactivation of prior known autoimmune disorders are reported. Though these reactions are rare, dermatologic complications are much more common, ranging up to 30–40% in all patients treated with PD-1 inhibitors; patients are estimated to be 2.6 times more likely to develop a rash after treatment with pembrolizumab than when undergoing standard chemotherapy2,5.
Generally, pembrolizumab has been shown to cause a maculopapular rash occurring on the trunk and extremities with facial sparing1. Though CTLA-4 related irAEs appear to be histologically consistent with CD4 infiltrates noted on biopsy, the pathology involved in PD-1 rashes is considerably more variegated: biopsies of pembrolizumab-attributed rashes in a study by Belum most closely resembled a lichenoid interface dermatitis2, but another study by Goldinger found the majority of their cutaneous anti-PD-1 reactions consisted of “a cytotoxic skin eruption characterized by an accumulation of CD8 T cells at the dermo-epidermal junction and CD8 T-cell exocytosis into the epidermis with apoptotic keratinocytes.”7 Pembrolizumab has also been associated with the development of vitiligo, erythema nodosum, and, in rare cases, bullous pemphigoid6,8–10.
Our patient had a known history of bullous pemphigoid prior to treatment. Bullous pemphigoid (BP) is an autoimmune blistering disorder characterized by tense, superficial, variably pruritic bullae consisting of clear fluid that generally develops on the flexor surfaces and abdomen of elderly patients11,12. Histopathologic exam yields acantholysis; IgG and C3 deposits are noted under direct immunofluorescence12. BP has been shown to resolve in response to glucocorticoid treatment. A 2016 study by Menzies found that patients with underlying autoimmune diseases such as psoriasis, rheumatoid arthritis, and Sjogren’s disease commonly developed exacerbations of their preexisting disease following anti-PD-1 therapy13, and prior history of BP may distinguish our patient from the other presentations cited here. In the majority of patients who develop these symptoms, severity was usually mild to moderate (Grade II-III13), but a small proportion of irAEs were severe enough to require discontinuation of the drug, as was true for our patient5.
Management of moderate to severe immunotherapy mediated bullous pemphigoid includes discontinuation of therapy and prompt initiation of systemic glucocorticoids, preferably prednisone at 1–2 mg/kg body weight. Treatment duration varies based on response to therapy, which can be up to 3–4 weeks, and is generally followed by prolonged taper. In steroid refractory cases, alternate immunosuppressive agents such as azathioprine, mycophenolate mofetil, methotrexate are recommended14,15.
In summary, despite the relatively low toxicity profile attributed to PD-1 inhibitors when compared to conventional chemotherapy, it is prudent to recognize these rare adverse toxicities. Prompt initiation of systemic glucocorticoids and discontinuation of immunotherapy is pivotal in the management.
REFERENCES
- 1.de Golian E, Kwong BY, Swetter SM, Pugliese SB. Cutaneous Complications of Targeted Melanoma Therapy. Curr Treat Options Oncol. 2016;17(11). doi: 10.1007/s11864-016-0434-0. [DOI] [PubMed] [Google Scholar]
- 2.Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60(2016):12–25. doi: 10.1016/j.ejca.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibney GT, Kudchadkar RR, DeConti RC, et al. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma. Clin Cancer Res. 2015;21(4):712–720. doi: 10.1158/1078-0432.CCR-14-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naidoo J, Schindler K, Querfeld C, et al. Autoimmune Bullous Skin Disorders with Immune Checkpoint Inhibitors Targeting PD-1 and PD-L1. Cancer Immunol Res. 2016:1–8. doi: 10.1158/2326-6066.CIR-15-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sibaud V, Meyer N, Lamant L, Vigarios E, Mazieres J, Delord JP. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28(4):254–263. doi: 10.1097/CCO.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 7.Goldinger SM, Stieger P, Meier B, et al. Cytotoxic Cutaneous Adverse Drug Reactions during Anti-PD-1 Therapy. Clin Cancer Res. 2016;22(16):4023–4029. doi: 10.1158/1078-0432.CCR-15-2872. [DOI] [PubMed] [Google Scholar]
- 8.Brunet-Possenti F, Mignot S, Deschamps L, Descamps V. Antiepidermis autoantibodies induced by anti-PD-1 therapy in metastatic melanoma. Melanoma Res. 2016;26(5):540–543. doi: 10.1097/CMR.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 9.G. C, R. A, S. C, A. C, P. F-P. A case of bullous pemphigoid in a patient with metastatic melanoma treated with pembrolizumab. Melanoma Res. 2015;25(3):265–268. doi: 10.1097/cmr.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 10.Wakade DV, Carlos G, Hwang SJE, Chou S, Hui R, Fernandez-Peñas P. PD-1 inhibitors induced bullous lichen planus-like reactions: A rare presentation and report of three cases. Melanoma Res. 2016;26(4):421–424. doi: 10.1097/CMR.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 11.Yancey KB, Lawley TJ. Immunologically Mediated Skin Diseases In: Kasper D, Fauci A, Hauser S, Longo D, Jameson JL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine, 19e New York, NY: McGraw-Hill Education; 2015. http://accessmedicine.mhmedical.com/content.aspx?aid=1120789844. [Google Scholar]
- 12.Kershenovich R, Hodak E, Mimouni D. Diagnosis and classification of pemphigus and bullous pemphigoid. Autoimmun Rev. 2014;13(4–5):477–481. doi: 10.1016/j.autrev.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2016;(September 2016):mdw443. doi: 10.1093/annonc/mdw443. [DOI] [PubMed] [Google Scholar]
- 14.Burton JL, Harman RR, Peachey RD, Warin RP et al. Azathioprine plus prednisone in treatment of pemphigoid. Br Med J. 1978. October 28;2(6146):1190–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eskin-Schwartz M, David M, Mimouni D et al. Mycophenolate mofetil for the management of autoimmune bullous diseases. Dermatol Clin. 2011 Oct;29(4):555–9. [DOI] [PubMed] [Google Scholar]
- 16.Friedman CF, Proverbs-Singh TA, and Postow MA, Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol, 2016. 2(10): p. 1346–1353. [DOI] [PubMed] [Google Scholar]