Figure 3 |. Irreversible inhibition of Hrr25.
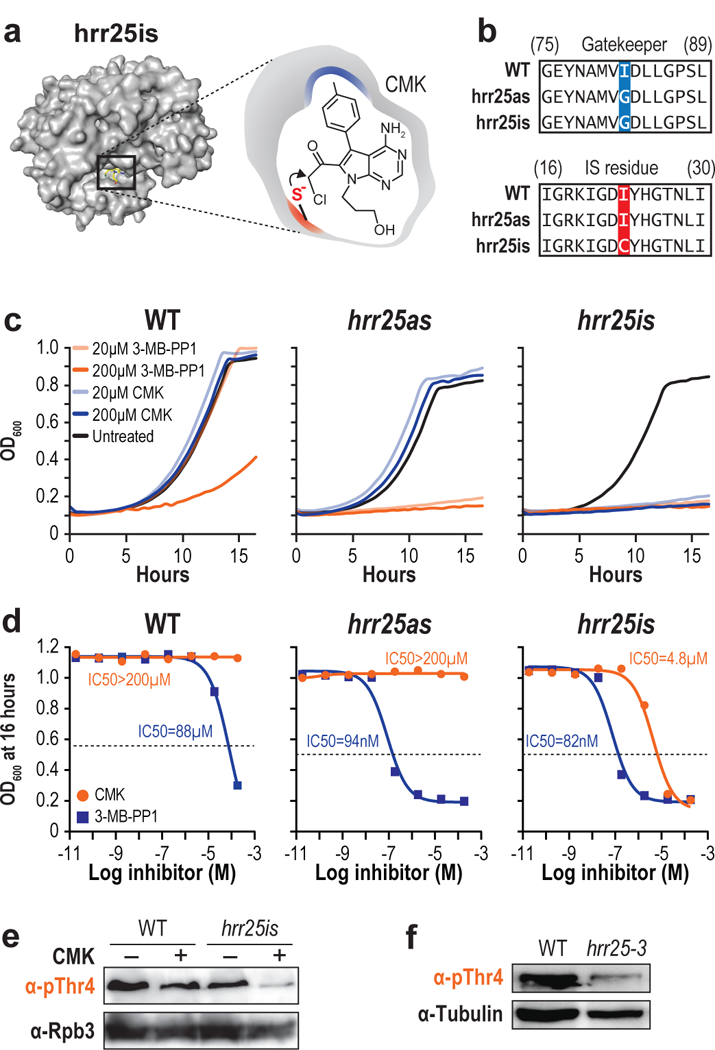
(a) Structure of hrr25is (left) and a schematic of CMK docking (right). (b) Sequence alignment of WT, hrr25as, and hrr25is with the gatekeeper substitution (blue) and “IS residue” substitution (red) shown. (c) Growth curves of WT (top), hrr25as (middle), or hrr25is (bottom) in the presence of DMSO (black), 20 μM 3-MB-PP1 (light orange), 200 μM 3-MB-PP1 (dark orange), 20 μM CMK (light blue), or 200 μM CMK (dark blue). (n = 3 independent experiments. Representative data from one experiment are shown.) (d) WT, Hrr2as, and Hrr25is cells were treated with either CMK or 3-MB-PP1 at concentrations ranging from 20 pM to 200 μM. For each concentration, the OD600 after 16 hours of inhibition is plotted. IC50 values were calculated with GraphPad Prism. (n = 2 independent experiments. Representative data from one experiment are shown.) (e) Western blots of yeast extract from WT or hrr25is cells treated with either DMSO (−) or CMK (+). Blots were probed with antibodies targeting pThr4 (1G7) or Rpb3 as a loading control. (n = 3 independent experiments). (f) Western blots of yeast extract from WT or hrr25–3 cells. Blots were probed with antibodies targeting pThr4 or Tubulin as a loading control. (n = 3 independent experiments). Full length blots are shown in Supplementary Fig. 12.
