Abstract
PURPOSE
We aimed to assess the association between features of epicardial adipose tissue and demographic, morphometric and clinical data, in a large population of symptomatic patients with clinical indication to cardiac computed tomography (CT) angiography.
METHODS
Epicardial fat volume (EFV) and adipose CT density of 1379 patients undergoing cardiac CT angiography (918 men, 66.6%; age range, 18–93 years; median age, 64 years) were semi-automatically quantified. Clinical variables were compared between diabetic and nondiabetic patients to assess potential differences in EFV and adipose CT density. Multiple regression models were calculated to find the clinical variables with a significant association with EFV and adipose CT density.
RESULTS
The median EFV in diabetic patients (112.87 mL) was higher compared with nondiabetic patients (82.62 mL; P < 0.001). The explanatory model of the multivariable analysis showed the strongest associations between EFV and BMI (β=0.442) and age (β=0.365). Significant yet minor association was found with sex (β=0.203), arterial hypertension (β=0.072), active smoking (β=0.068), diabetes (β=0.068), hypercholesterolemia (β=0.046) and cardiac height (β=0.118). The mean density of epicardial adipose tissue was associated with BMI (β=0.384), age (β=0.105), smoking (β=0.088), and diabetes (β=0.085).
CONCLUSION
In a large population of symptomatic patients, EFV is higher in diabetic patients compared with nondiabetic patients. Clinical variables are associated with quantitative features of epicardial fat.
Epicardial adipose tissue (EAT) is a fat storage tissue located beneath the pericardium (Fig. 1), representing approximately 15% of the cardiac weight (1, 2).
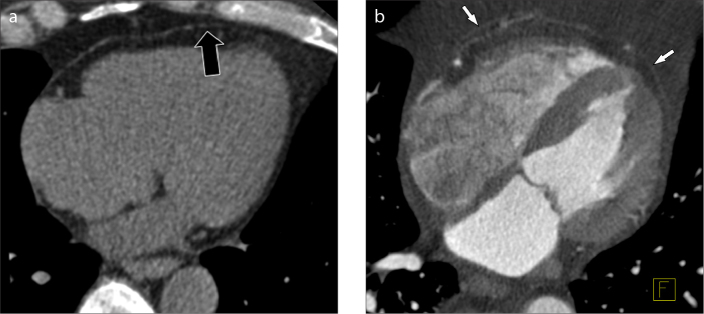
Figure 1. a, b.
Native (a) and contrast-enhanced (b) multiplanar reformatted (MPR) CT images of the heart showing epicardial fat (black arrow) and pericardium (white arrows).
The physiologic distribution of EAT is characterized by major representation in the atrioventricular septa and interventricular sulcus, where it surrounds coronary arteries (3). EAT is a visceral fat that secretes inflammatory mediators, and it acts as paracrine promotor of atherosclerosis by means of adipokines and proinflammatory cytokines (e.g., monocyte chemotactic protein 1, interleukin 6, and tumor necrosis factor α) (4). Such local modulators drive the chemotaxis of inflammatory cells into the arterial wall leading to chronic vascular remodelling, notably in coronary arteries (1, 5, 6). Several studies showed the association between epicardial fat volume (EFV) and atherosclerotic plaques associated with higher risk of cardiovascular events (1, 7–11).
Epicardial adipose tissue can be measured with different imaging techniques: ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI) (12, 13). Cardiac CT angiography (CCTA) with volumetric acquisition shows the highest spatial resolution and reproducibility for quantitative analysis of EFV, including the assessment of the CT attenuation coefficient (14–16). A number of studies described EFV features mainly in asymptomatic subjects, with a minor proportion of symptomatic subjects. Such heterogeneity is not ideal for the stratification of cardiovascular risk, which accounts also for symptoms, beyond physiologic and diagnostic parameters (5, 9).
Among traditional cardiovascular risk factors, diabetes mellitus (DM) is associated with mortality from systemic atherosclerosis (17). Noteworthy, diabetic patients were shown to carry an increased amount of EAT (18, 19). However, this association was not confirmed in every study evaluating EFV and clinical characteristics (20, 21). A retrospective study showed a 7.4% prevalence of DM in Parma residents which is higher than the age-standardized prevalence for individuals living in the neighbouring areas of Northern Italy (i.e., 4%) (22). The Alternative Cardiovascular Bio-Imaging markers (ALTER-BIO) registry comprises symptomatic individuals referred at the University Hospital of Parma because of cardiovascular symptoms. This large registry could provide information about the distribution of EFV in symptomatic individuals and, in particular, between diabetic and nondiabetic individuals.
The purpose of this study was to compare EFV and clinical characteristics between diabetic and nondiabetic subjects, in a large population of patients with clinical indication to CCTA and a relatively high prevalence of DM. Furthermore, we aimed to test the association between the characteristics of EAT and the demographic, morphometric and clinical data of a homogeneous population of symptomatic individuals.
Methods
Patients selection
The patients who underwent CCTA between October 2006 and November 2010 at the University Hospital of Parma (Parma, Italy) were retrospectively retrieved from the picture archiving and communication system (PACS). The study has been approved by the Institutional Review Board of the University Hospital of Parma and written informed consent was waived. Selection criteria were as follows: A) suspicion for obstructive coronary artery disease (CAD) based on clinical and instrumental data (symptoms included typical or atypical chest pain, asthenia, dyspnoea, palpitations, arrhythmias, syncope and neurological manifestations, i.e., headache, transient ischemic attack) (23); B) availability of volumetric CCTA dataset for quantitative measurements; C) availability of demographic and biometric parameters, including sex, age, smoking habit, and body mass index (BMI), patients with BMI ≥30 kg/m2 considered obese (24); D) availability of the following clinical parameters: a) DM defined by fasting hyperglycemia, HbA1c ≥6.5% (25), or glucose lowering therapy; b) arterial hypertension defined by systolic arterial pressure ≥140 mmHg or diastolic arterial pressure ≥90 mmHg, or antihypertensive therapy (26); c) cardiovascular medical history as follows: previous cardiac disease, broadly defined acute coronary syndrome (ACS), broadly defined known vascular disease (e.g., aortic dissection, abdominal aortic aneurysm, subarachnoid hemorrhage), and family history of CAD – including acute myocardial infarction or sudden cardiac death – reported in a 1st degree relative; d) blood analysis including serum creatinine, lipid profile (HDL-cholesterol, LDL-cholesterol, and triglycerides), and glycemia. Presence of hypercholesterolemia, hypertriglyceridemia, and hypolipemic therapy was retrieved from the medical charts.
Exclusion criteria was the presence of previous coronary revascularization (percutaneous or surgical).
CCTA procedure
CCTAs with electrocardiographic gating were acquired with two multidetector scanners: a 64-slice Somatom Sensation Cardiac scanner (Siemens Medical Solutions) and a 128-slice Somatom Definition Flash scanner (Siemens Medical Solutions). Before the scan, a sublingual tab of nitroglycerine (0.3 mg) was administered to provide transient coronary dilation. Patients presenting with heart rate >60 beats per minute were administered 5 mg of intravenous atenolol under electrocardiographic and pressure monitoring, provided the absence of contraindication to the administration of β-blocker (e.g., asthma, bronchospasm, or systolic arterial pressure values <100 mmHg). Images were acquired before and after the intravenous injection of iodinated contrast agent (Iomeron 400, Bracco; 80–120 mL apportioned to the body weight, injection rate 3.5–5 mL/s) through the antecubital vein followed by a saline chaser (50 mL), using a double syringe injector. The acquisition of the angiographic phase was automatically triggered by opacification of the ascending aorta ≥100 HU.
CCTA quantitative analysis
EFV was independently measured by two operators with a quantitative semi-automated procedure using a postprocessing workstation (MMWP, Siemens Medical Solutions) and its segmentation software (Volume) (14, 27). The operator manually traced 8 to 10 regions of interest (ROI) along the margins of the fibrous pericardium at different levels on cardiac axial slices, from the pulmonary valve to the lowest slice with a detectable pericardium. The ROIs were interpolated by a segmentation algorithm based on densitometric threshold (density range between −190 HU and −30 HU). Then, the selected volume was visually reviewed with multiplanar reconstructions and manual editing, until the definition of the optimal outline of EFV (expressed in mL) (28) (Figs. 2 and 3). The upper normal limit for EFV was set at 100 mL, as previously proposed by Sarin et al. (14).
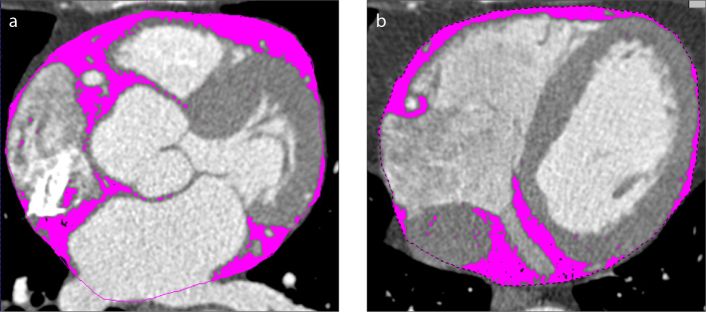
Figure 2. a, b.
Magnifications of a cardiac CT angiography image in the axial view (at two different anatomical levels) show the segmentation process of epicardial fat (pink areas) performed by the quantification software. The pink dotted line represents the region of interest.
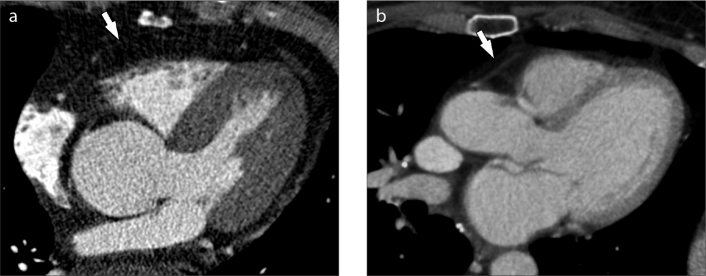
Figure 3. a, b.
Multiplanar reformatted (MPR) CT images of two different patients (a, b). The pericardium is highlighted by the white arrows. The two patients display different values of EFV, with higher amount in image (a).
The software calculated also the mean density of the EAT (expressed in HU) and its standard deviation (SD), and cardiac height (namely, the distance between the upper and lower slice within the segmented volume, expressed in cm).
Coronary artery calcifications (CAC) were quantified by a dedicated semi-automated software (CaScore, Siemens Medical Solutions) for segmentation of dense areas in the coronary artery (density threshold > 130 HU) (Fig. 4). The overall CAC score for each patient was calculated using the Agatston score algorithm (29). CAC scores were stratified into 4 groups (group 0: 0; group 1: 1–100; group 2: 101–300; group 3: >400).
Figure 4.
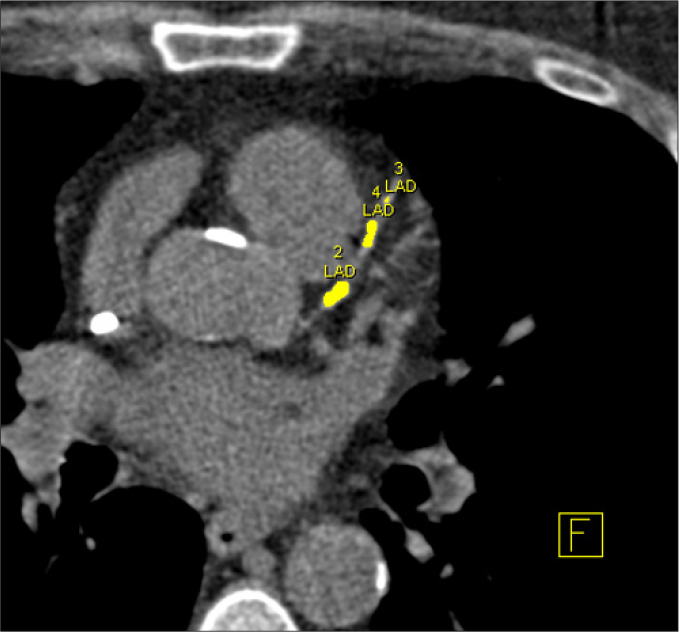
Quantification of coronary artery calcifications. The left anterior descending coronary artery is highlighted in yellow and the total amount of calcium is assessed by a dedicated software as the sum of pixel with density above 130 HU.
Statistical analysis
The coefficient of variation was calculated to assess interobserver reproducibility between the operators involved in measuring EFV (27). The interobserver variability was assessed by the intraclass correlation coefficient (ICC) for continuous variables. Furthermore, Lin’s concordance correlation coefficient (CCC) acted as index of reproducibility between the operators’ measurements and the mean of each paired measurement (30). According to McBride et al. (31), we considered almost perfect agreement at CCC >0.990 (31). Normality of data distribution was assessed by Shapiro-Wilk test. Normally distributed variables were reported as mean and SD, non-normally distributed variables were reported as median and interquartile range (IQR). Appropriate comparison tests were used for parametric variables and for nonparametric variables. Chi-square test was used to compare categorical variables. All variables were compared between diabetic and nondiabetic patients to assess differences in conventional clinical data and potential difference in EFV. Given the absence of outliers with an excessive influence on models, Pearson’s univariate correlation coefficient was used. Finally, multiple regression model was used, including all the clinically relevant variables that showed significance at the univariate analysis. Only variables with a variance inflation factor <2 were included to test the multicollinearity. The EFV was evaluated as logarithmic value (logVol) to normalize the residuals and to keep the whole population sample. For such models, standardized coefficients were reported instead of the significance. A P value ≤ 0.05 was deemed statistically significant. The statistical analysis was performed by statistical software IBM SPSS Statistics, 23 (IBM Corp.).
Results
The coefficient of variation for measurements of EFV performed in 250 patients was 9%. ICC was excellent (0.980, 95%CI 0.963–0.988). Lin’s CCC was 0.995, namely above the threshold of excellent agreement, for both operators. Therefore, the remaining CCTAs were independently read by either operator.
EFV was measured for 1379 patients with clinical indication to CCTA. Demographic and clinical information are listed in Table 1. Three clinical parameters were available for only a portion of the dataset, as follows: lipid-lowering therapy (available for 1307/1379 patients, 95%), triglycerides levels (available for 539/1379 patients, 39%), and HDL-cholesterol levels (available for 403/1379 patients, 29%). Lipid-lowering therapy by statins was administered to 40.2% of patients (525/1307), of whom 92% (483/525) had hypercholesterolemia, and 13.9% (73/525) had hypertriglyceridemia. CAC were quantified on 1.351 patients (median CAC score 58, IQR, 452), the distribution was as follows: 388 (28.7%) in group 0, 344 (25.5%) in group 1, 253 (18.7%) in group 2, and 366 (27.1%) in group 3.
Table 1.
Demographic and clinical features of patients
| Clinical feature | |
|---|---|
| Male gender, n (%) | 918 (66.6) |
|
| |
| Age (years), median (IQR) | 64 (18) |
|
| |
| Smoking history, n (%) | |
| Never smoker | 797 (57.8) |
| Former smoker | 279 (20.2) |
| Current smoker | 303 (22) |
|
| |
| BMI (kg/m2), median (IQR) | 26.7 (5.33) |
|
| |
| Obesity, n (%) | 297 (21.5) |
|
| |
| DM, n (%) | 338 (24.5) |
|
| |
| Hypertension, n (%) | 953 (69.1) |
|
| |
| Previous ACS, n (%) | 250 (18.1) |
|
| |
| Hypercholesterolemia, n (%) | 777 (56.3) |
|
| |
| Hypertriglyceridemia, n (%) | 145 (10.5) |
IQR, interquartile range; BMI, body mass index; DM, diabetes mellitus; ACS, acute coronary syndrome.
The comparison of the clinical characteristics between diabetic and nondiabetic patients is summarized in Table 2, reporting the results of chi-square tests. EFV >100 mL was recorded in 63.6% (215/338) of diabetic and 36.6% (381/1041) of nondiabetic patients (P < 0.001) (14). Diabetic patients were older (P < 0.001), had higher BMI (P < 0.001), and were more frequently obese (P < 0.001); furthermore, they suffered from arterial hypertension (P < 0.001), hypercholesterolemia (P = 0.050), and showed more frequently a medical history of ACS (P = 0.042).
Table 2.
Clinical features of the patients according to the diabetic status
| Clinical features | Diabetic patients | Nondiabetic patients | P |
|---|---|---|---|
| Epicardial fat volume (median, 91.46 mL) | |||
| Below median | 99 (29.3) | 451 (43.3) | < 0.001 |
| Above median | 239 (70.7) | 590 (56.7) | |
|
| |||
| Gender | |||
| Male | 226 (66.9) | 692 (66.5) | 0.947 |
| Female | 112 (33.1) | 349 (33.5) | |
|
| |||
| Age (median, 64 years) | |||
| Below median | 137 (40.5) | 591 (56.8) | < 0.001 |
| Above median | 201 (59.5) | 450 (43.2) | |
|
| |||
| Body mass index (median, 26.27 kg/m2) | |||
| Below median | 91 (26.9) | 543 (52.2) | < 0.001 |
| Above median | 247 (73.1) | 498 (47.8) | |
|
| |||
| Arterial hypertension | |||
| Yes | 274 (81.1) | 679 (65.2) | < 0.001 |
| No | 64 (18.9) | 362 (34.8) | |
|
| |||
| Patients with previous acute coronary syndrome | |||
| Yes | 74 (21.9) | 176 (16.9) | 0.042 |
| No | 264 (78.1) | 865 (83.1) | |
|
| |||
| Vasculopatic patients | |||
| Yes | 65 (19.2) | 225 (21.6) | 0.398 |
| No | 273 (80.8) | 816 (78.4) | |
|
| |||
| Hypercholesterolemia | |||
| Yes | 206 (60.9) | 571 (54.9) | 0.050 |
| No | 132 (39.1) | 470 (45.1) | |
|
| |||
| Hypertriglyceridemia | |||
| Yes | 37 (10.9) | 108 (10.4) | 0.766 |
| No | 301 (89.1) | 933 (89.6) | |
|
| |||
| Smoking history | |||
| Nonsmoker | 202 (59.8) | 595 (57.2) | 0.411 |
| Former/current smoker | 136 (40.2) | 446 (42.8) | |
Looking at the comparisons involving quantitative variables, EFV was significantly higher in diabetic patients (range, 21.37–442.21; median, 112.87; IQR, 68.07) compared with nondiabetic patients (range, 11.27–317.99; median, 82.62; IQR, 62.17) (Mann Whitney U test, P < 0.001). Furthermore, the mean density of EAT was lower in diabetic patients (−80.78±6.06 HU) as compared to nondiabetic patients (−78.19±5.27 HU; independent-samples t-test, P < 0.001). Higher CAC scores were seen in diabetic individuals (P < 0.001) and EFV was higher in diabetic patients with greater CAC scores (P = 0.001).
Considering the overall sample, there was a positive correlation between EFV and CAC scores (ρ=0.343, P < 0.001). Finally, there was a negative correlation between densitometric value and both EFV (ρ= −0.634, P < 0.001) and BMI (ρ= −0.438, P < 0.001). In particular, between EAT density and EFV there was a similar negative correlation for both diabetic and nondiabetic patients (ρ= −0.615, P < 0.001 and ρ= −0.613, P < 0.001, respectively).
The multivariable analysis developed explanatory models for EFV (adjusted R2=0.475, P < 0.001) and EAT mean density (adjusted R2=0.241, P < 0.001). For EFV, the strongest associations were found with BMI (β=0.442) and age (β=0.365). Other significant associations were found with sex (β=0.203), arterial hypertension (β=0.072), active smoking (β=0.068), DM (β=0.068), hypercholesterolemia (β=0.046), and cardiac height (β=0.118) (Table 3).
Table 3.
Association between clinical variables and epicardial fat volume and CT-based epicardial adipose tissue mean density
| Coefficients | Epicardial fat volumea (mL) | Epicardial adipose tissueb (mean HU) | ||
|---|---|---|---|---|
| β | P | β | P | |
| Constant | < 0.001 | < 0.001 | ||
| Diabetes mellitus | 0.068 | 0.001 | −0.085 | 0.001 |
| Cardiac height | 0.118 | < 0.001 | 0.162 | < 0.001 |
| Age | 0.365 | < 0.001 | −0.105 | < 0.001 |
| Gender | 0.203 | < 0.001 | −0.031 | 0.203 |
| Body mass index (BMI) | 0.442 | 0.001 | −0.384 | < 0.001 |
| Smoking history | 0.068 | 0.001 | −0.088 | < 0.001 |
| Hypertension | 0.072 | 0.001 | −0.040 | 0.125 |
| Hypercholesterolemia | 0.046 | 0.026 | −0.033 | 0.181 |
| Hypertriglyceridemia | −0.016 | 0.433 | 0.024 | 0.325 |
| Vasculopathy | 0.016 | 0.421 | 0.005 | 0.846 |
| Previous acute coronary syndrome | 0.015 | 0.448 | 0.011 | 0.655 |
Epicardial fat volume is expressed as logarithmic value.
Epicardial adipose tissue mean density is expressed as Hounsfield Unit.
Similarly, the mean density of EAT was associated with BMI (β=0.384) and age (β=0.105). Significant, yet minor, association was found with smoking (β=0.088), DM (β=0.085), and cardiac height (β=0.162) (Table 3).
Discussion
In a population of patients with clinical indication to CCTA, an excess of EAT was seen in diabetic patients, independently from other morphometric and clinical cardiovascular risk factors. These results set the role of DM in the balance of visceral fat deposit and supports the role of EAT as a metabolically active tissue with quantitative modifications due to dysmetabolic conditions, namely DM.
In keeping with prior studies, we have shown that the quantification of EFV by CT is reproducible (27). The independent quantification was particularly beneficial because it allowed a reduction of the overall time required for the read-out of the large study population. The same observation can be translated to implementation of this measurement to clinical practice. Of note, quantitative measurements of EAT did not require additional radiation exposure, as they were obtained from datasets acquired for clinical practice.
Compared with the resident population of Parma, our study sample included a greater prevalence of DM (24.5% vs. 7.4%) (22). This high prevalence allowed us to expand upon the relationship between EAT and DM by focusing on patients with cardiovascular symptoms referred to CT evaluation of a suspected obstructive CAD. Mahabadi et al. (9) showed that EFV was associated with DM in 4093 individuals, with a prevalence of 12.4% diabetics. Furthermore, they reported that EFV was directly associated with the presence of cardiovascular risk factors, in individuals without medical history of CAD, acute myocardial infarction, and cardiac surgery (9). Konishi et al. (32) showed positive correlation between pericardial fat volume and markers of DM in patients with suspected CAD, and a prevalence of 33% diabetics over 171 subjects. Wang et al. (33) reported that EFV was higher in 49 diabetic patients compared with 78 nondiabetic controls. In a population of 402 patients, EFV was higher in men with arterial hypertension, hypercholesterolemia, and smokers, but not in patients with DM (20). Similarly, Bos et al. (21) reported in a large population of patients that DM was not related with EFV in the multivariate analysis, whereas there was a significant relation in the univariate analysis. Our multivariable analysis showed a significant association between DM and EFV, notably in a large population with a remarkable component of diabetic patients. Further parameters associated with increased EFV were age, sex, BMI, smoking, arterial hypertension, and hypercholesterolemia. We postulate that the association between EFV and age may partially include the effect of DM toward an increase of EFV, as older individuals show the highest prevalence of DM (34). Indeed, clinical characteristics of the metabolic syndrome (e.g., obesity, dyslipidemia, and hypertension) were significantly associated with EFV, probably acting as confounders for EFV (35). Iacobellis et al. (36) showed association between EAT and impaired insulin sensitivity as well as fasting glucose, and Gorter et al. (27) reported a significant association between EFV and metabolic syndrome, underlining a tight correlation between the systemic disease and the conspicuity of visceral fat and its metabolic activity. Yorgun et al. (37) showed that the strongest independent variables related with EAT thickness were metabolic syndrome, BMI, and age. Furthermore, they reported that serum triglyceride levels were not correlated with an increased EAT thickness. Previous studies demonstrated the presence of a correlation between EFV and triglycerides (32, 38). However, the degree of correlation was wide. Dong et al. (39) found a very weak correlation between the two variables. Furthermore, Mookadam et al. (40) did not find association between triglycerides an EAT thickness on echocardiography, and Hell et al. (41) reported that hypertension was the only significant cardiovascular risk factor for EFV and EAT density. In our study, we hypothesize that the lack of correlation in the multivariable analysis between EFV and hypertriglyceridemia could be related to the administration of lipid-lowering therapy in 452 patients with normal levels of circulating triglycerides (452/525, 86.1%) at the moment of the scan; therefore, statins might have biased triglyceride levels causing the lack of correlation between EFV and triglycerides (42).
Currently, there is not consensus for the normal range of EFV (14, 28). It was reported that EFV was positively associated with multiple cardiovascular risk factors, with a significant association with metabolic syndrome in patients with EFV >100 mL (14, 38, 43, 44). In patients with clinical indication to CCTA, we report median EFV of 82.62 mm3 in nondiabetics and of 112.87 mm3 in diabetics. Diabetic patients were significantly more represented above the 100 mL threshold of EFV, conversely the nondiabetic patients were mostly below.
In our study population, diabetic individuals had higher CAC scores than nondiabetic individuals. This observation is in keeping with previous studies reporting a higher frequency of CAC, a complication of atherosclerotic lesions, in diabetic individuals (45). Diabetic individuals are at risk for accelerated atherosclerosis and Wang et al. (33) suggested that the association between EAT, metabolic syndrome, and atherosclerosis could be related by a common pathway for obesity, adiposity, metabolic syndrome, and inflammation. We found that individuals with higher EFV had higher CAC scores, this was in keeping with previous reports from asymptomatic populations (21, 46, 47). The association between EFV and CAC is a subject of current debate as either direct or indirect mechanism. Mahabadi et al. (48) postulated that shared risk factors could explain the increasing amount of CAC in patients with higher EFV. Nevertheless, a systematic review from Spearman et al. (10) proposed that EAT surrounding coronary arteries may be a determinant of atherosclerosis, arterial stiffness and CAC, but the mechanism is not fully understood yet.
Several studies showed that EAT density values on CT may vary according to histologic features. In particular, adipose tissue with higher HU shows a lower amount of intracellular lipids and a richer vascularization (49). The adipocytes’ hypertrophy was associated with increased pro-inflammatory macrophages (50). An increased attenuation value was related to the fibrosis of the adipose tissue’s extracellular matrix (51). In our study, EAT density and EFV were negatively correlated for both diabetic and nondiabetic patients. This correlation is consistent with the results of Mahabadi et al. (52), who reported a modest, yet significant, inverse correlation between these two morphometric parameters. We showed that diabetic patients had lower EAT density and higher EFV. We hypothesize that the adipose tissue in diabetic individuals could be more frequently characterized by hypertrophic adipocytes (with lower EAT density) and, potentially, associated high level of proinflammatory macrophages. Such a pattern might be a contributor to the increased cardiovascular risk of diabetic patients, notably via the paracrine effect of visceral fat reservoir in tight proximity to coronary arteries.
Our study presents some limitations. First, as a cross-sectional study we could evaluate only associations and not causality. Second, it was not possible to obtain full clinical information for a minority of the study patients owing to the retrospective study fashion. Anamnestic and demographic information were retrieved from radiology reports and from the review of medical charts; however, for three clinical parameters, data were available for only a portion of the study population.
In conclusion, our results show that EFV is increased in diabetic patients compared with nondiabetic, in the subset of symptomatic patients. In particular, higher EFV in diabetics might add to systemic mediators of endothelial stress and enhance coronary vasculopathy by means of increased paracrine metabolic activity of EAT. On this basis, further analyses are fostered on the possible association between the CT characteristics of EAT and CT markers of coronary disease, in both diabetics and nondiabetics.
Main points.
Cardiac CT angiography can quantitatively assess epicardial fat volume (EFV) and CT density.
EFV is higher in diabetic patients compared with nondiabetic patients.
Epicardial fat density is lower in diabetic patients compared with nondiabetic patients.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Marwan M, Achenbach S. Quantification of epicardial fat by computed tomography: why, when and how? J Cardiovasc Comput Tomogr. 2013;7:3–10. doi: 10.1016/j.jcct.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 3.Aldiss P, Davies G, Woods R, Budge H, Sacks HS, Symonds ME. ‘Browning’ the cardiac and peri-vascular adipose tissues to modulate cardiovascular risk. Int J Cardiol. 2017;228:265–274. doi: 10.1016/j.ijcard.2016.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 5.Ding J, Hsu FC, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2009;90:499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 7.Aslanabadi N, Salehi R, Javadrashid A, et al. Epicardial and pericardial fat volume correlate with the severity of coronary artery stenosis. J Cardiovasc Thorac Res. 2014;6:235–239. doi: 10.15171/jcvtr.2014.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahabadi AA, Reinsch N, Lehmann N, et al. Association of pericoronary fat volume with atherosclerotic plaque burden in the underlying coronary artery: a segment analysis. Atherosclerosis. 2010;211:195–199. doi: 10.1016/j.atherosclerosis.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Mahabadi AA, Berg MH, Lehmann N, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol. 2013;61:1388–1395. doi: 10.1016/j.jacc.2012.11.062. [DOI] [PubMed] [Google Scholar]
- 10.Spearman JV, Renker M, Schoepf UJ, et al. Prognostic value of epicardial fat volume measurements by computed tomography: a systematic review of the literature. Eur Radiol. 2015;25:3372–3381. doi: 10.1007/s00330-015-3765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cademartiri F, Sverzellati N, Guaricci AI, Maffei E. Fat and cardiovascular risk: the role of Cardiac CT. Eur Heart J Cardiovasc Imaging. 2016;17:1368–1369. doi: 10.1093/ehjci/jew193. [DOI] [PubMed] [Google Scholar]
- 12.Dey D, Nakazato R, Li D, Berman DS. Epicardial and thoracic fat - Noninvasive measurement and clinical implications. Cardiovasc Diagn Ther. 2012;2:85–93. doi: 10.3978/j.issn.2223-3652.2012.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim BJ, Kim BS, Kang JH. Echocardiographic epicardial fat thickness is associated with arterial stiffness. Int J Cardiol. 2013;167:2234–2238. doi: 10.1016/j.ijcard.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Sarin S, Wenger C, Marwaha A, et al. Clinical significance of epicardial fat measured using cardiac multislice computed tomography. Am J Cardiol. 2008;102:767–771. doi: 10.1016/j.amjcard.2008.04.058. [DOI] [PubMed] [Google Scholar]
- 15.Alvey NJ, Pedley A, Rosenquist KJ, et al. Association of fat density with subclinical atherosclerosis. J Am Heart Assoc. 2014:3. doi: 10.1161/JAHA.114.000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Grutta L, Toia P, Farruggia A, et al. Quantification of epicardial adipose tissue in coronary calcium score and CT coronary angiography image data sets: comparison of attenuation values, thickness and volumes. Br J Radiol. 2016;89:20150773. doi: 10.1259/bjr.20150773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyle PJ. Diabetes mellitus and macrovascular disease: mechanisms and mediators. Am J Med. 2007;120(Suppl 2):S12–17. doi: 10.1016/j.amjmed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Christensen RH, von Scholten BJ, Hansen CS, et al. Epicardial, pericardial and total cardiac fat and cardiovascular disease in type 2 diabetic patients with elevated urinary albumin excretion rate. Eur J Prev Cardiol. 2017 doi: 10.1177/2047487317717820. 2047487317717820. [DOI] [PubMed] [Google Scholar]
- 19.Levelt E, Pavlides M, Banerjee R, et al. Ectopic and visceral fat deposition in lean and obese patients with type 2 diabetes. J Am Coll Cardiol. 2016;68:53–63. doi: 10.1016/j.jacc.2016.03.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajani R, Shmilovich H, Nakazato R, et al. Relationship of epicardial fat volume to coronary plaque, severe coronary stenosis, and high-risk coronary plaque features assessed by coronary CT angiography. J Cardiovasc Comput Tomogr. 2013;7:125–132. doi: 10.1016/j.jcct.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bos D, Shahzad R, van Walsum T, et al. Epicardial fat volume is related to atherosclerotic calcification in multiple vessel beds. Eur Heart J Cardiovasc Imaging. 2015;16:1264–1269. doi: 10.1093/ehjci/jev086. [DOI] [PubMed] [Google Scholar]
- 22.Del Canale S, Louis DZ, Maio V, et al. The relationship between physician empathy and disease complications: an empirical study of primary care physicians and their diabetic patients in Parma, Italy. Acad Med. 2012;87:1243–1249. doi: 10.1097/ACM.0b013e3182628fbf. [DOI] [PubMed] [Google Scholar]
- 23.Gregoratos G. Clinical manifestations of acute myocardial infarction in older patients. Am J Geriatr Cardiol. 2001;10:345–347. doi: 10.1111/j.1076-7460.2001.00641.x. [DOI] [PubMed] [Google Scholar]
- 24.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(Suppl 2):S102–138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002;48:436–472. doi: 10.2337/diacare.25.4.750. [DOI] [PubMed] [Google Scholar]
- 26.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 27.Gorter PM, van Lindert AS, de Vos AM, et al. Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis. 2008;197:896–903. doi: 10.1016/j.atherosclerosis.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Shmilovich H, Dey D, Cheng VY, et al. Threshold for the upper normal limit of indexed epicardial fat volume: derivation in a healthy population and validation in an outcome-based study. Am J Cardiol. 2011;108:1680–1685. doi: 10.1016/j.amjcard.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-T. [DOI] [PubMed] [Google Scholar]
- 30.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. doi: 10.2307/2532051. [DOI] [PubMed] [Google Scholar]
- 31.McBride GB. Using statistical methods for water quality management: issues, problems and solutions. 2005. Available from: https://www.health.govt.nz/system/files/documents/publications/equivalence-measures-2007.pdf.
- 32.Konishi M, Sugiyama S, Sugamura K, et al. Association of pericardial fat accumulation rather than abdominal obesity with coronary atherosclerotic plaque formation in patients with suspected coronary artery disease. Atherosclerosis. 2010;209:573–578. doi: 10.1016/j.atherosclerosis.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Wang CP, Hsu HL, Hung WC, et al. Increased epicardial adipose tissue (EAT) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin Endocrinol (Oxf) 2009;70:876–882. doi: 10.1111/j.1365-2265.2008.03411.x. [DOI] [PubMed] [Google Scholar]
- 34.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650–2664. doi: 10.2337/dc12-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beltran-Sanchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J Am Coll Cardiol. 2013;62:697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iacobellis G, Leonetti F. Epicardial adipose tissue and insulin resistance in obese subjects. J Clin Endocrinol Metab. 2005;90:6300–6302. doi: 10.1210/jc.2005-1087. [DOI] [PubMed] [Google Scholar]
- 37.Yorgun H, Canpolat U, Hazirolan T, et al. Increased epicardial fat tissue is a marker of metabolic syndrome in adult patients. Int J Cardiol. 2013;165:308–313. doi: 10.1016/j.ijcard.2011.08.067. [DOI] [PubMed] [Google Scholar]
- 38.Jang HC, Lee HK, Lee H, Cha JG, Kim YS, Cho JH. Analyzing correlation between epicardial fat area and metabolic syndrome risk factor by using low-dose Lung CT. Pak J Med Sci. 2015;31:1207–1212. doi: 10.12669/pjms.315.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong DD, Wang K, Wang D, Zhang T, Tu YF, Shen BZ. Relationship between epicardial adipose tissue volume measured using coronary computed tomography angiography and atherosclerotic plaque characteristics in patients with severe coronary artery stenosis. J Int Med Res. 2013;41:1520–1531. doi: 10.1177/0300060513496169. [DOI] [PubMed] [Google Scholar]
- 40.Mookadam F, Goel R, Alharthi MS, Jiamsripong P, Cha S. Epicardial fat and its association with cardiovascular risk: a cross-sectional observational study. Heart Views. 2010;11:103–108. doi: 10.4103/1995-705X.76801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hell MM, Ding X, Rubeaux M, et al. Epicardial adipose tissue volume but not density is an independent predictor for myocardial ischemia. J Cardiovasc Comput Tomogr. 2016;10:141–149. doi: 10.1016/j.jcct.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 42.Ginsberg HN. Effects of statins on triglyceride metabolism. Am J Cardiol. 1998;81:32B–35B. doi: 10.1016/S0002-9149(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 43.Iacobellis G, Ribaudo MC, Assael F, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5163–5168. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 44.Lehman SJ, Massaro JM, Schlett CL, O’Donnell CJ, Hoffmann U, Fox CS. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: the Framingham Heart Study. Atherosclerosis. 2010;210:656–661. doi: 10.1016/j.atherosclerosis.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yahagi K, Kolodgie FD, Lutter C, et al. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2017;37:191–204. doi: 10.1161/ATVBAHA.116.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bettencourt N, Toschke AM, Leite D, et al. Epicardial adipose tissue is an independent predictor of coronary atherosclerotic burden. Int J Cardiol. 2012;158:26–32. doi: 10.1016/j.ijcard.2010.12.085. [DOI] [PubMed] [Google Scholar]
- 47.Dey D, Wong ND, Tamarappoo B, et al. Computer-aided non-contrast CT-based quantification of pericardial and thoracic fat and their associations with coronary calcium and metabolic syndrome. Atherosclerosis. 2010;209:136–141. doi: 10.1016/j.atherosclerosis.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng VY, Dey D, Tamarappoo B, et al. Pericardial fat burden on ECG-gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovasc Imaging. 2010;3:352–360. doi: 10.1016/j.jcmg.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu HH, Chung SA, Nayak KS, Jackson HA, Gilsanz V. Differential computed tomographic attenuation of metabolically active and inactive adipose tissues: preliminary findings. J Comput Assist Tomogr. 2011;35:65–71. doi: 10.1097/RCT.0b013e3181fc2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spencer M, Unal R, Zhu B, et al. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab. 2011;96:E1990–1998. doi: 10.1210/jc.2011-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahabadi AA, Balcer B, Dykun I, et al. Cardiac computed tomography-derived epicardial fat volume and attenuation independently distinguish patients with and without myocardial infarction. PLoS One. 2017;12:e0183514. doi: 10.1371/journal.pone.0183514. [DOI] [PMC free article] [PubMed] [Google Scholar]