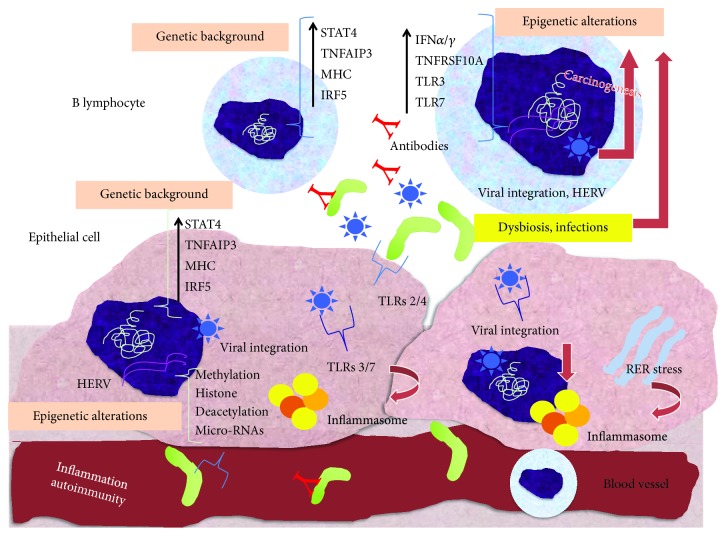
Figure 1.
Pathogenesis of Sjögren's syndrome and associated B cell lymphoma. On the basis of a favourable genetic background, represented by the hyperexpression of genes related to the inflammatory cascade (STAT4, TNFAIP3, MHC, and IRF5) shared by both B lymphocytes and salivary gland epithelial cells, infections or dysbiosis may modify the cell epigenetic machinery and consequently control gene expression, favouring inflammation, autoimmunity, and lymphomagenesis. Particularly, the epigenetic induction of the transcription of genes associated to microbial recognition (e.g., TLR3 and TLR7) and IFN-I signature, by means of aberrant methylation, histone deacetylation, and micro-RNA expression, may further promote the hyperactivation of B cells in response to foreign or endogenous epitopes, generated by bacteria, viruses, and endogenous retroelements. In addition, RER stress and integrated retroelements may favour the activation of inflammasomes and cause cell DNA rearrangements, which represent crucial steps for chronic B cell activation and transformation. These steps finally converge to the hyperproduction of autoantibodies cross-reacting with antigens locally or systemically, inflammation and destruction of glandular tissues, and B cell transformation into malignant clones. STAT4: signal transducer and activator of transcription 4; TNFAIP3: tumor necrosis factor-alpha-induced protein 3; MHC: major histocompatibility complex; IRF5: interferon-regulatory factor 5; IFN: interferon; TNFRSF10: tumor necrosis factor receptor superfamily member 10A; TLR: Toll-like receptors; HERV: human endogen retroviruses; RER: rough endoplasmic reticulum.