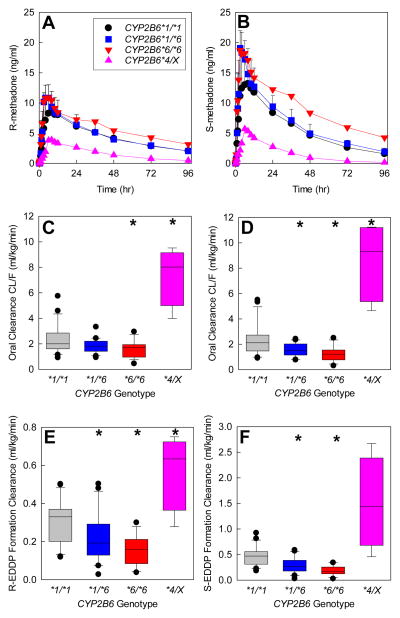
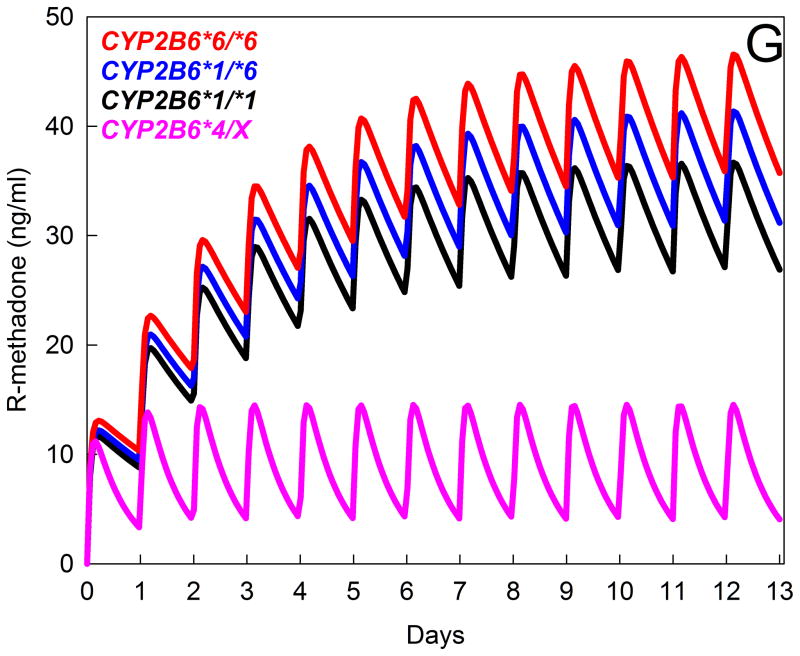
Figure 5.
Influence of CYP2B6 genotype on the disposition and metabolism of oral RS-methadone. Genotype cohorts were CYP2B6*1/*1 (n=21), CYP2B6*1/*6 (n=20), CYP2B6*6/*6 (n=17), and CYP2B6*4/X (n=4, with results for one CYP2B6*1/*4 and three CYP2B6*4/*6 subjects combined). *Significantly different from wild-type (CYP2B6*1/*1), P < 0.05. EDDP = 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine, CL/F = apparent oral clearance.
(A and B) Plasma concentrations of R- and S-methadone after a single oral dose (mean ± SD, some SD values omitted for clarity). Reproduced from Kharasch et al39 with permission.
(C and D) Apparent oral clearance of R-methadone (C) and S-methadone (D). Results are shown as box plots (solid line within the box represents the median, dashed line within the box represents the mean, box boundaries are the 25th and 75th percentiles, error bars are the 10th and 90th percentiles, and individual points are outliers). Redrawn from Kharasch et al39 with permission.
(E and F) R- and S-EDDP formation clearance. Results are shown as box plots. Redrawn from Kharasch et al39 with permission.
(G) Influence of CYP2B6 genetic polymorphisms on plasma R-methadone concentrations during daily dosing, predicted from single-dose data. Multi-dose methadone enantiomer plasma concentrations were predicted for the various CYP2B6 genotypes using public-access models (http://copnt13.cop.ufl.edu/safezone/pat/pha5127/simulatn.htm) and the pharmacokinetic parameters obtained previously (apparent oral clearance, volume of distribution, bioavailability),39 and a reported oral absorption constant.42 Result are shown (predicted plasma R-methadone concentrations) for once-daily dosing of 20 mg oral racemic methadone (10 mg R-methadone). Simulations do not incorporate any influence of autoinduction of methadone clearance over the first 2 weeks of dosing, which is known to occur,42 and would decrease plasma concentrations during the second week and thereafter, because the relative susceptibility of various CYP2B6 genotypes to autoinduction is not known.