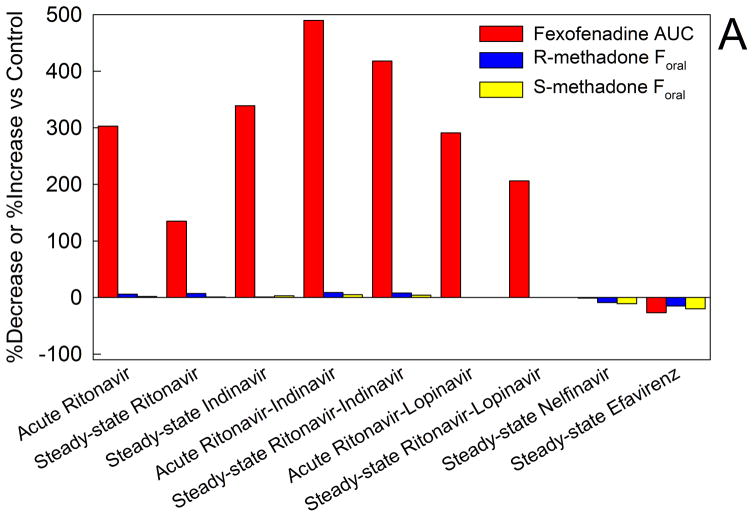
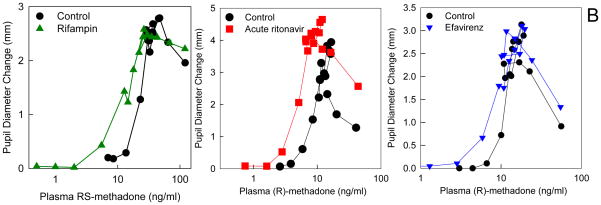
Figure 6.
Methadone disposition and clinical effects and drug transporters. (A) Effect of HIV antiretrovirals on intestinal transporter activity and methadone bioavailability. Transporter (P-glycoprotein) activity was assessed using oral fexofenadine as a substrate probe. Results are from a series of drug interaction studies in healthy volunteers. For all of the methadone-antiretroviral interaction studies fexofenadine and methadone disposition were determined in the same subjects on different days.17–23 Acute antiretroviral effects were determined after 2–3 days, and steady-state effects were determined after two weeks. Results are shown as the average per cent increase (ascending bars) or decrease (descending bars) in fexofenadine plasma area under the curve (AUC) and the bioavailability of oral methadone (Foral). Increased fexofenadine plasma AUC is interpreted as inhibition of intestinal P-glycoprotein efflux activity and increased drug absorption.
(B) Effect of HIV antiretrovirals and an antituberculosis drug on methadone pharmacodynamics (plasma concentration-effect relationships). Subjects simultaneously received intravenous and also oral methadone (accounting for the second concentration peak, 3–4 h after the initial intravenous peak, due to slow absorption of oral methadone). Results (representing the sum of intravenously and orally administered methadone) are shown for RS-methadone or for the more pharmacologically active R-methadone enantiomer. Methadone effect was determined from the decrease in pupil diameter (miosis). Leftward and/or upward shifts in the concentration-effect relationship suggest increased apparent potency or efficacy and increased brain methadone concentrations. Each data point is the mean of 12 subjects, with SDs omitted for clarity. Data are redrawn from previous publications16,18,23 with permission.