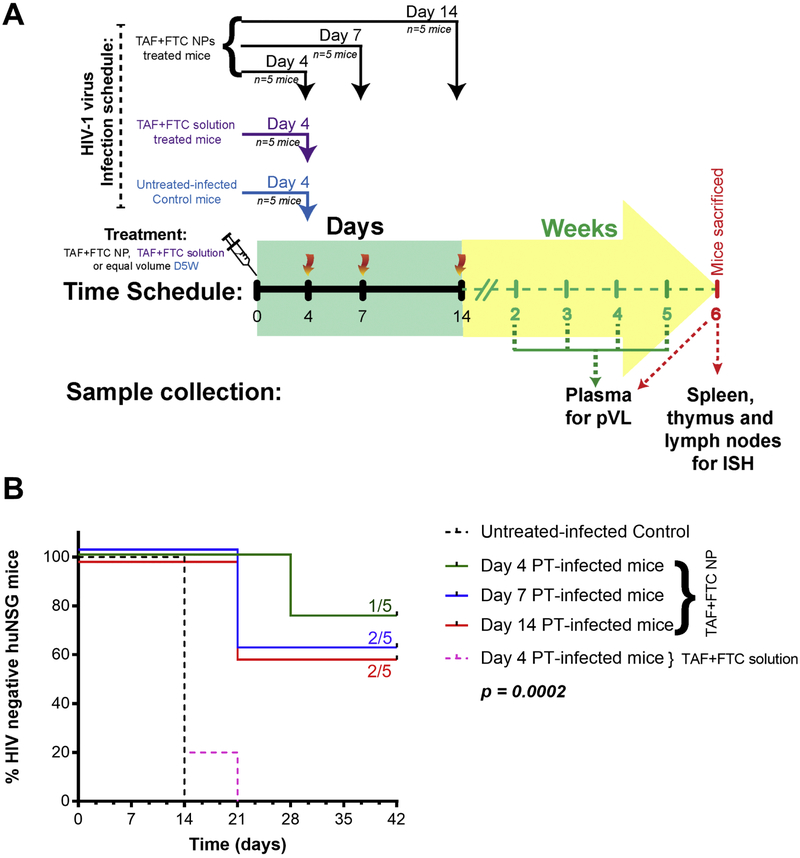
Figure 3.
The protection study design schedule (A) and the Kaplan-Meier infectivity curve (B) of Hu-BLT mice receiving each drug at 200 mg/kg as TAF+FTC NPs or as TAF+FTC solution. Each mice group (n=5) were intravaginal challenged with T/F HIV-1 strain on 4, 7, or 14 days after PT of TAF+FTC NPs. The untreated-infected control mice group (n=5) and TAF+FTC solution group (n=5), were challenged on Day 4 post-SubQ treatment (PT).