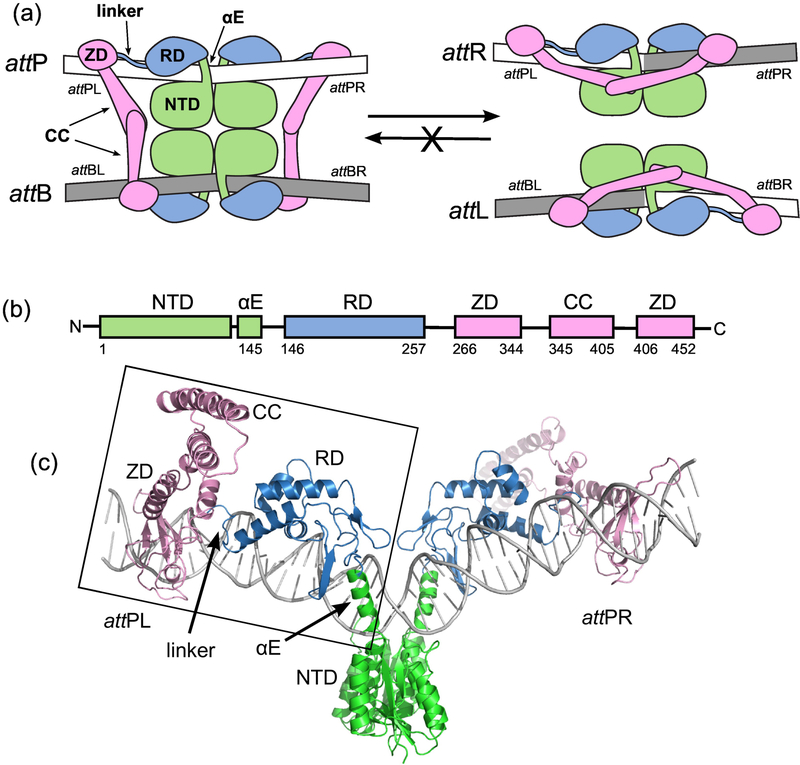
Figure 1.
The integration reaction carried out by serine integrases. A) Schematic of the overall reaction. Integrase dimers bind to attP and attB and associate the sites to form the complex shown on the left. Site-specific recombination catalyzed by the N-terminal catalytic domains (NTDs) results in the hybrid sites attL and attR (right). The reverse reaction that converts attL and attR to attP and attB does not occur at a measurable rate in the absence of a phage encoded RDF protein. For detailed descriptions of the catalytic mechanism, see [2,5]. attPL and attPR refer to the left and right half-sites of attP. B) Domain structure of LI Int. αE is an alpha helix that extends from the NTD to the recombinase domain (RD). A coiled-coil (CC) motif is embedded in the zinc ribbon domain (ZD). C) Structural model of the Int-attP complex. The boxed region corresponds to the experimental LI CTD•attPL crystal structure and has been duplicated to model the full site [25]. The nature of the bend at the center of the site is not currently known.