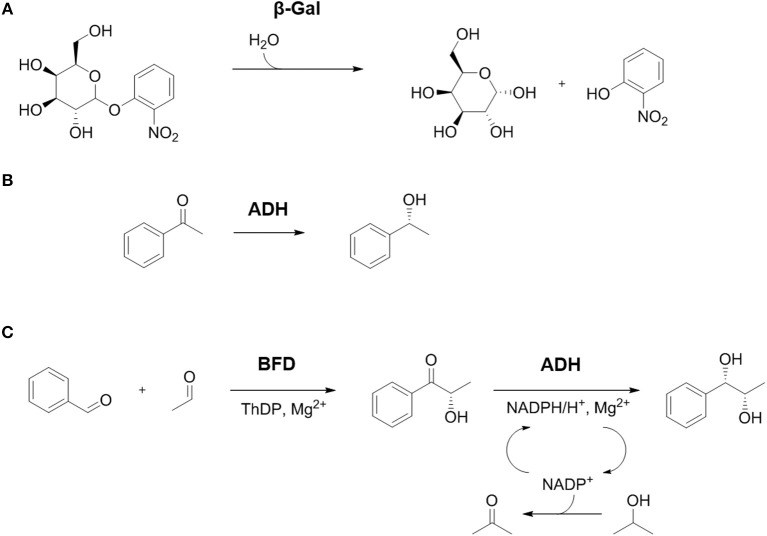
Figure 1.
Overview of the studied reactions. (A) Hydrolysis of o-Nitrophenyl-β-d-galactopyranoside (ONPG) by β-Gal yields the monosaccharides galactose and o-nitrophenol. (B) Activity of ADH can be determined by enantioselective reduction of acetophenone to (R)-phenylethanol. (C) The combination of BFD and ADH in a 2-step cascade reaction. Carboligation of the educts benzaldehyde and acetaldehyde catalyzed by BFD yields the intermediate (S)-2-hydroxy-1-phenyl-propanone ((S)-HPP), which can be further reduced to the product (1S,2S)-1-phenylpropane-1,2-diol ((S,S)-PPD) by ADH. The redox equivalents are delivered by the cofactor NADPH that is oxidized to NADP+. For in situ regeneration of NADPH 2-propanol was added in excess which is oxidized to acetone by the same ADH.