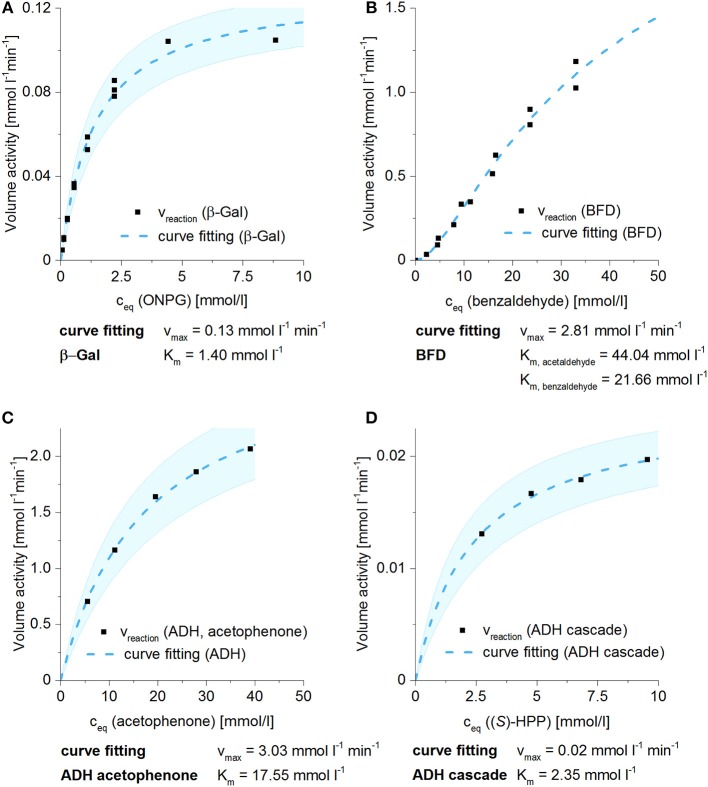
Figure 3.
Michaelis–Menten kinetics of the investigated biocatalytic reactions with freely dissolved enzymes. Kinetic parameters vmax and Km were calculated by fitting the data to the Michaelis–Menten function, which is shown including 95% confidence bounds. In the whole figure, each data point represents one sample. (A) β-Gal kinetics for the cleavage of ONPG at pH 4.6 (n = 3 separate runs). (B) BFD kinetics for the carboligation of benzaldehyde and a respective 2.5-fold excess of acetaldehyde (n = 2 separate runs). Estimation of the confidence bounds was omitted because of limited data for substrate excess. (C) ADH kinetics for the reduction of acetophenone. (D) ADH kinetics for the reduction of (S)-HPP, which was previously synthesized by BFD. For ADH kinetics, data points were generated in one batch due to shortage of the enzyme.