Abstract
Microbial interactions represent an understudied facet of human health and disease. In this study, the interactions that occur between Chlamydia trachomatis and the opportunistic fungal pathogen, Candida albicans were investigated. Candida albicans is a common component of the oral and vaginal microbiota responsible for thrush and vaginal yeast infections. Normally, Candida exist in the body as yeast. However, disruptions to the microbiota create conditions that allow expanded growth of Candida, conversion to the hyphal form, and tissue invasion. Previous studies have shown that a myriad of outcomes can occur when Candida albicans interacts with pathogenic bacteria. To determine if C. trachomatis physically interacts with C. albicans, we incubated chlamydial elementary bodies (EB) in medium alone or with C. albicans yeast or hyphal forms for 1 h. Following incubation, the samples were formaldehyde-fixed and processed for immunofluorescence assays using anti-chlamydial MOMP or anti- chlamydial LPS antibodies. Replicate samples were replenished with culture medium and incubated at 35°C for 0–120 h prior to fixation for immunofluorescence analysis or collection for EB infectivity assays. Data from this study indicates that both C. trachomatis serovar E and C. muridarum EB bind to C. albicans yeast and hyphal forms. This interaction was not blocked by pre-incubation of EB with the Candida cell wall components, mannan or β-glucans, suggesting that EB interact with a Candida cell wall protein or other structure. Bound EB remained attached to C. albicans for a minimum of 5 days (120 h). Infectivity assays demonstrated that EB bound to C. albicans are infectious immediately following binding (0h). However, once bound to C. albicans, EB infectivity decreased at a faster rate than EB in medium alone. At 6h post binding, 40% of EB incubated in medium alone remained infectious compared to only 16% of EB bound to C. albicans. Likewise, pre-incubation of EB with laminarin, a soluble preparation of β-glucan, alone or in combination with other fungal cell wall components significantly decreases chlamydial infectivity in HeLa cells. These data indicate that interactions between EB and C. albicans inhibit chlamydial infectivity, possibly by physically blocking EB interactions with host cell receptors.
Keywords: Chlamydia trachomatis, Candida albicans, bacterial-fungal interactions, co-culture, normal flora, opportunistic infections
Introduction
Trillions of commensal microbes reside in the human body at all times. While a diverse microbiota has been correlated with health, some studies indicate that microbial interactions can alter the pathogenesis of infection (Baquero and Nombela, 2012; D’Argenio and Salvatore, 2015; Krezalek et al., 2016; Ziklo et al., 2016). For example, respiratory viruses create an environment that is favorable for the growth of bacterial opportunistic pathogens, leading to infections such as otitis media and bacterial pneumonia (McCullers, 2006; Armbruster et al., 2010). Co-infection with sexually transmitted pathogens can increase the transmission of HIV (Rotchford et al., 2000). Thus, interactions between pathogenic and/or commensal microbes represent an understudied facet of human health and disease. In this study, we examined interactions between the bacterial pathogen, Chlamydia trachomatis and the opportunistic fungal pathogen, Candida albicans.
Chlamydia trachomatis is the leading bacterial sexually transmitted infection, causing 1.6 million reported cases in the United States in 2016 (Centers for Disease Control and Prevention, 2017). Genital infections with Chlamydia are usually benign with many being asymptomatic or causing uncomplicated cervicitis in women and urethritis in men. However, if untreated these infections can ascend the genital tract leading to pelvic inflammatory disease, ectopic pregnancy and infertility (Peipert, 2003). Chlamydia are Gram-negative obligate intracellular bacteria, which exist in the host in two distinct morphological forms. Transmission occurs when the infectious, condensed, extracellular form, the elementary body (EB) enters into the host cell by endocytosis. Once inside the host cell the EB convert to the non-infectious, intracellular, metabolically active, vegetative form called the reticulate body (RB). The RB replicate inside of a modified host vacuole called the inclusion before converting back into EB. Infectious EB are released from the host cell by host cell lysis or extrusion and lysis of the inclusion (Elwell et al., 2016).
Candida albicans is a common opportunistic pathogen in the oral and vaginal microbiota and is responsible for vaginal yeast infections and thrush (Mayer et al., 2013). Normally, C. albicans exist in the body as a yeast member of the fungal microbiome. However, when given the opportunity, such as in the case of immunosuppression or antibiotic usage, C. albicans can convert to an invasive hyphal form that damages the mucosal tissue (Mayer et al., 2013). Invasive Candida infections can lead to life threatening fungal sepsis in immunosuppressed individuals. Candida have a complex cell wall. The outer layer of the Candida cell wall is comprised of mannose polymers, called mannans, that are bound to proteins embedded in the cell wall. The inner layer of the cell wall is primarily composed of β-1,3-glucan and β-1,6-glucan. A small portion of the cell wall is composed of chitin. These structures are critical to the structural integrity of the C. albicans cell wall in both the yeast and hyphal forms. Additionally, both mannans and β-glucans are immune stimulatory molecules that are recognized by a variety of host pattern recognition receptors, especially C-type lectin receptors (Gow et al., 2012).
Several previous studies have shown that both symbiotic and antagonistic Candida/bacterial interactions occur depending upon the environmental conditions and species involved. Pseudomonas aeruginosa, Escherichia coli, and Acinetobacter baumannii as well as several other bacteria have been shown to inhibit growth of Candida hyphal forms by varying known and unknown mechanisms (Hogan and Kolter, 2002; Peleg et al., 2008, 2010). For example, Pseudomonas aeruginosa forms biofilms on Candida albicans hyphae, but cannot bind to yeast (Hogan and Kolter, 2002). P. aeruginosa releases several soluble compounds including phospholipase C, phenazines and quorum sensing molecules that inhibit the growth of Candida hyphal forms, decreasing Candida’s virulence (Morales et al., 2013). Conversely, Candida sp. inhibit the growth of Neisseria gonorrheae via an unknown mechanism (Kaye and Levison, 1977). Fungal/bacterial interactions can also increase microbial pathogenesis. Simultaneous infections of E. coli and C. albicans in mice increased lethality compared to single infections with either organism (Gale and Sandoval, 1957; Peleg et al., 2010). Interaction of Streptococcus sp., including pathogenic Group B Streptococcus, with Candida promotes enhanced biofilm formation and adhesion to host epithelia (Peleg et al., 2010; Pidwill et al., 2018). Binding of Staphylococcus to Candida hyphae promotes dissemination of the bacteria into the blood stream of mice (Peters et al., 2012; Kong et al., 2015). Other studies suggest that Candida can serve as a protective reservoir for Helicobacter pylori, which has been detected in some, albeit not all, instances of atherosclerosis and Alzheimer’s disease (Honjo et al., 2009; Saniee and Siavoshi, 2015). Thus, several interesting outcomes have been documented with C. albicans/bacterial interactions. These interactions have the potential to: (1) inhibit growth/virulence of Candida or the bacterial pathogens, (2) provide a reservoir for bacterial survival within the body, or (3) promote bacterial dissemination from the site of mucosal infection.
Kelly et al. (2001) demonstrated that concurrent infection with C. albicans and Chlamydia muridarum did not alter the immune response or vaginal shedding of either organism in mice. However, this study did not investigate direct interaction of the two pathogens in vitro or in a simultaneous in vivo inoculation (Kelly et al., 2001). Interestingly, dissemination of Chlamydia sp. from the site of primary infection to distal sites in the body has been reported. Chlamydial DNA has been isolated from the synovial fluid of patients suffering from Chlamydia-induced reactive arthritis (Baillet et al., 2015). Dissemination of Chlamydia pneumoniae has been associated with atherosclerosis and Alzheimer’s disease (Kuo and Campbell, 2003; Shima et al., 2010). The mechanisms chlamydiae use to disseminate from the site of primary infection throughout the body have not been fully investigated. Given the myriad of medically relevant outcomes that have been reported from investigations of Candida/bacterial interactions and the high probability that Candida and Chlamydia encounter one another in host genital tracts, we sought to determine if direct interactions between C. trachomatis and C. albicans occur.
Materials and Methods
Culture of Candida, Chlamydia, and Cervical Epithelial Cell Lines
Candida albicans SC5314 cultures were routinely cultured overnight at 30°C in YPD (1%yeast extract, 2% peptone, 2% dextrose). Overnight cultures of C. albicans were washed with sterile dH2O and counted before transferring cells to medium 199 [M199 (9.5 g medium with Earles salts and L-glutamine and without sodium bicarbonate, 18.7 g Tris-HCL in 1L dH2O, pH 7.5 or pH 4.5) [Mediatech] at 37°C. Chlamydia trachomatis Serovar E and C. muridarum Weiss stocks were prepared in Hec-1B cells grown in bead culture as previously described (Guseva et al., 2007). HeLa cells were maintained in Modified Eagle’s Medium (MEM; Gibco) supplemented with 10% FBS, and gentamicin (Gibco). Short tandem repeat profiling was performed by the American Type Culture Collection to authenticate the identity and/or origin of all cell lines used in this study (data not shown). All cell lines were tested for Mycoplasma by PCR and found to be free of contamination (data not shown).
Mannan, β-Glucan, and Laminarin
Fungal cell wall components, mannan, glucan phosphate (β-glucan) or laminarin were prepared in ddH2O (1 mg/ml). Mannan was isolated from Candida albicans (Kruppa et al., 2011). The β-glucan phosphate was a kind gift from Dr. David Williams at East Tennessee State University. It was prepared from Saccharomyces cerevisiae (Williams et al., 1991). Laminarin was purchased from Carbosynth, United States. Purification and activity validation of these biomolecules have been previously described (Williams et al., 1991; Adams et al., 2008; Kruppa et al., 2011; Smith et al., 2018).
Candida/Chlamydia Binding Assay
Candida albicans SC5314 (1 × 105 cells/sample) was cultured as yeast for 3–6 h in M199 (pH 4.5) medium at 37°C. The yeast were then mixed with 3 × 105 C. trachomatis Serovar E or C. muridarum EB or 2SPG (0.2 M sucrose, 0.02 M phosphate buffer, and 5 mM l-glutamine) and plated onto coverslips. Alternatively, C. albicans was cultured on FBS-coated plastic coverslips for 3–6 h in M199 (pH7.5) medium at 37°C to promote hyphal formation. The Candida cultures or plastic coverslips were overlaid with 3x105 EB or 2SPG. Following combination of Candida yeast or hyphal cultures with EB, the samples were incubated at 35°C for 1 h. The cultures were then washed vigorously three times with PBS to remove unbound EB and fixed with methanol, formaldehyde or glutaraldehyde. In a subset of experiments, EB were overlaid onto plastic coverslips and incubated for 1 h at 35°C, but the inoculum was not removed from the well following incubation. This no-wash (NW) control was replenished with the appropriate medium as described below and harvested for EB titer analysis. Replicate samples were replenished with culture medium (M199 ± 5 μg/ml Amphotericin B or M199 or MEM ± 1 mM glucose-6-phosphate/1% FBS) and incubated for 24–120 h before fixation for IFA or harvest for EB titer analysis. In some experiments, EB were pre-incubated (1 h, 37°C) with fungal cell wall components (1 mg/ml), mannan, glucan phosphate (β-glucan) or laminarin alone or in combination prior to incubation with Candida cultures.
Immunofluorescence Assays and Confocal Microscopy Analysis
Methanol or formaldehyde-fixed samples were stained with BioRad anti-MOMP or LPS Pathfinder stain or with a mouse anti-Chlamydia MOMP antibody (Abcam BIOD)/rabbit-anti-mouse Alexa Fluor 488 combination. C. albicans was either stained with aniline blue or visualized by differential interference contrast (DIC) microscopy. Samples were examined and imaged using a Zeiss Axiovert microscope and Zen 2012 software. Intensity of fluorescent staining/area (um2) was measured by imaging 5 random fields from triplicate experiment samples (n = 15) at 200× magnification using set exposure conditions. Confocal images of chlamydial inclusions were captured on a Leica TCS SP8 with Leica LASX software at 1000× magnification. Imaris imaging and Leica LASX software were used to analyze the images.
Transmission Electron Microscopy
Candida albicans or C. albicans/C. trachomatis cultures were fixed with glutaraldehyde in 0.1 M Cacodylate buffer (EM Sciences) for 24 h. The monolayers were then collected, agar enrobed, and stained with osmium tetroxide and uranyl acetate before alcohol and propylene oxide dehydration (Wyrick et al., 1994). Dehydrated samples were embedded in Spurr’s low viscosity resin (EM Sciences Kit 14300) to improve penetration of the medium into the Candida cell wall (Taff and Andes, 2013). Thin sections were examined on a Tecnai 10 (FEI) transmission electron microscope operating at 60–80 kV.
Percent Infectivity
Elementary bodies were pre-incubated (1 h, 37°C) in 2SPG alone or containing 1 mg/ml fungal cell wall components, mannan, β-glucan, or laminarin alone or in combination. Following exposure to the cell wall components, HeLa 229 cells were inoculated with the EB/cell wall component mixtures for 1h at 35°C. The inoculum was aspirated from the infected cultures and the cells were replenished with MEM+10% FBS. At 48hpi, the samples were fixed with methanol and stained for chlamydial inclusions using Pathfinder anti-Chlamydia MOMP stain (BioRad). Cell nuclei were stained with DAPI (ThermoFisher). Chlamydial inclusions and host cell nuclei were visualized on a Zeiss Axiovert Discovery Microscope at 400× magnification. In each experiment, the number of inclusions and nuclei were counted in 15 random fields from triplicate samples and the average percentage of infected cells was calculated per condition. A high dilution of EB was used in these studies to make counting the percentage of infected cells more accurate.
EB Titer Analysis
Triplicate C. albicans, C. trachomatis, C. albicans/C. trachomatis cultures, prepared as described in section “Candida/Chlamydia Binding Assay”, were scraped into the culture medium and frozen at -80°C. EB released from the cells by freeze/thaw and sonication were diluted in culture medium (MEM + 10%FBS, 0.5 μg/ml cycloheximide, gentamicin, and 5 μg/ml amphotericin B) and used to infect HeLa 229 monolayers grown on coverslips by spin infection (1 h, 1100 ×g). Triplicate infected monolayers were incubated for 48 h at 35°C before methanol fixation and staining with Pathfinder anti-Chlamydia MOMP stain (BioRad). Total inclusions were counted on each coverslip using a Zeiss Axiovert Discovery Microscope at 200x magnification. The average number of inclusion forming units (IFU)/ml was calculated per sample and experimental condition.
Statistical Analysis
All experiments contained three biological replicates and were independently repeated a minimum of three times. Values from independent experiments were averaged and presented as the mean ± the standard error of the mean (SEM). Means were compared by Analysis of Variance (ANOVA) and independent two-sample T-tests using MiniTab Version 17 Statistical Software and Microsoft Excel. Comparisons with p-values ≤ 0.05 were considered significantly different.
Results
Chlamydia Elementary Bodies Bind to the Surface of Candida albicans Yeast and Hyphal Forms
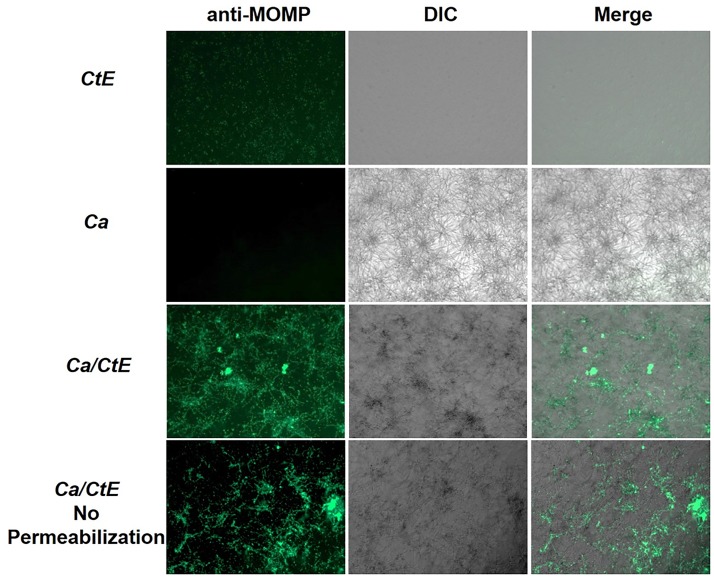
We sought to determine if Chlamydia EB physically interacts with C. albicans by examining EB binding to yeast or hyphal forms. We first assessed EB binding to Candida hyphal forms. Candida yeast or medium alone was seeded onto FBS-coated coverslips and incubated for 3h under conditions that promote hyphal formation. The Candida cultures were then overlaid with C. trachomatis serovar E EB or 2SPG such that Chlamydia (CtE), Candida (Ca) and Candida albicans/Chlamydia trachomatis (Ca/CtE) samples were prepared. Following incubation, the cultures were washed, replenished with medium and incubated for 24h before fixation with methanol or formaldehyde and processed for IFA to visualize any elementary bodies (EB) bound to the coverslips or Candida. These experiments demonstrated that EB bind to Candida hyphal cultures and remain bound for 24h (Figure 1). No significant difference in chlamydial staining was observed between samples permeabilized with methanol or not permeabilized, indicating that the observed fluorescence was primarily due to binding of EB on the surface of C. albicans (Figure 1, bottom row). A similar result was observed with C. muridarum EB binding to C. albicans (Supplementary Figure 1). In replicate experiments, Ca/CtE samples were harvested for analysis by confocal and transmission electron microscopy as described in the methods. For confocal microscopy we stained Candida with the non-specific stain, aniline blue. As shown in Figure 2A, EB staining co-localized with the aniline blue staining of Candida hyphae. The chlamydia staining indicates that the EB are bound to the surface of C. albicans as there was a clear separation in the Chlamydia and Candida staining observed in the digital sections and in cross-sections of Ca/CtE samples (Figures 2B,C). TEM images also demonstrate that EB (Figures 2E–G, yellow arrows) bind to the surface of the Candida cell wall (Figures 2D–G, white arrows). Data from these imaging analyses support the IFA data, which indicate that Chlamydia EB bind to the surface of C. albicans hyphae.
FIGURE 1.
Chlamydia trachomatis EB bind to the surface of Candida albicans. FBS-coated coverslips were inoculated with C. albicans or medium alone and incubated 3 h prior to exposure to EB (Ca/CtE or CtE) or 2SPG (Ca). Following incubation of 1 h, the cultures were washed, replenished with medium and incubated for 24 h before collection for IFA by fixation with methanol or formaldehyde (No permeabilization). Samples were stained with Pathfinder anti-Chlamydia MOMP stain and visualized 100× magnification.
FIGURE 2.
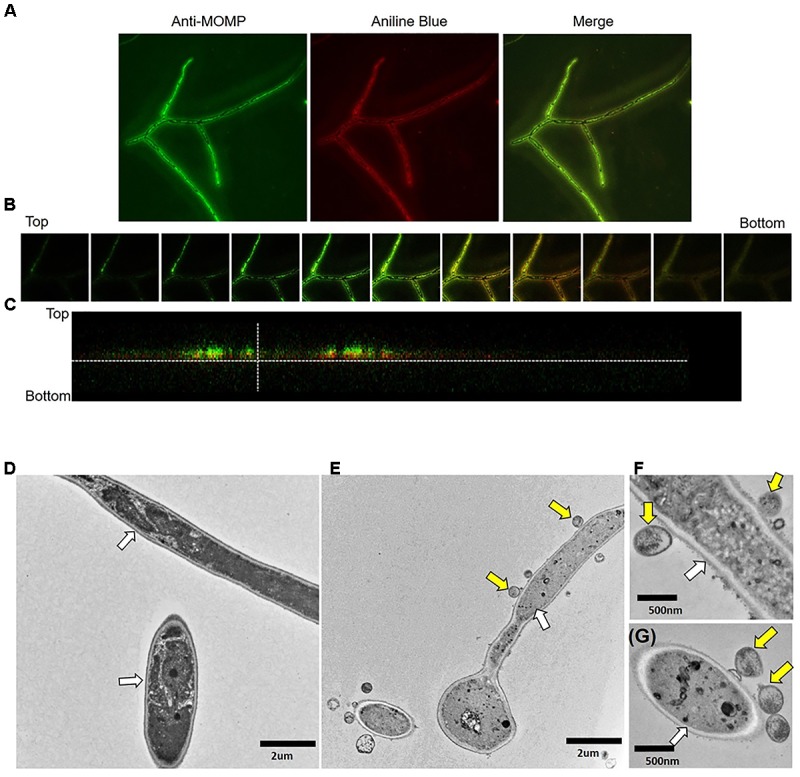
Confocal and transmission electron microscopy imaging of C. albicans/C. trachomatis cultures. C. albicans hyphal cultures were prepared alone or exposed to EB and processed for IFA and TEM visualization as described in the methods. (A) Confocal image of Ca/CtE culture at 100x magnification. (B) Individual Z sections taken at 30 micron intervals throughout the depth of the sample. Bottom denotes the coverslip. (C) Cross-section of the confocal image presented in (A). Green: Pathfinder anti-Chlamydia MOMP stain, Red: Aniline blue stain for Candida. (D) TEM micrograph of Ca culture at 7000× magnification. (E) TEM micrograph of Ca/CtE culture at 7000× magnification. (F,G) TEM micrographs of Ca/CtE cultures at 20000× magnification. Yellow Arrows: C. trachomatis EB, White Arrows: Candida cell wall.
As C. albicans usually exists in vivo in the yeast form, we examined the ability of EB to bind to C. albicans yeast. Yeast cultures or medium alone was mixed with EB or 2SPG in suspension for 1h prior to plating the samples onto FBS-coated coverslips. The yeast were then allowed to attach to the coverslips for 1h before the cultures were washed, replenished with medium, and incubated for 0–120 h (1–5 days) before fixation and IFA analysis. In duplicate samples we repeated the Candida hyphae/EB binding study described above, harvesting the cultures 0–120 h (1–5 days) for IFA analysis. In both experiments, a replicate set of Ca/CtE cultures were exposed to Amphotericin B to inhibit overgrowth of the Candida biofilm. Data from these studies indicate that EB bound to both C. albicans yeast and hyphal forms and remained bound for a minimum of 5 days (120 h, Supplementary Figure 2). Continued binding was most evident in samples in which new Candida growth was limited by Amphotericin B.
Chlamydia Elementary Bodies Bound to the Surface of Candida albicans Lose Infectivity
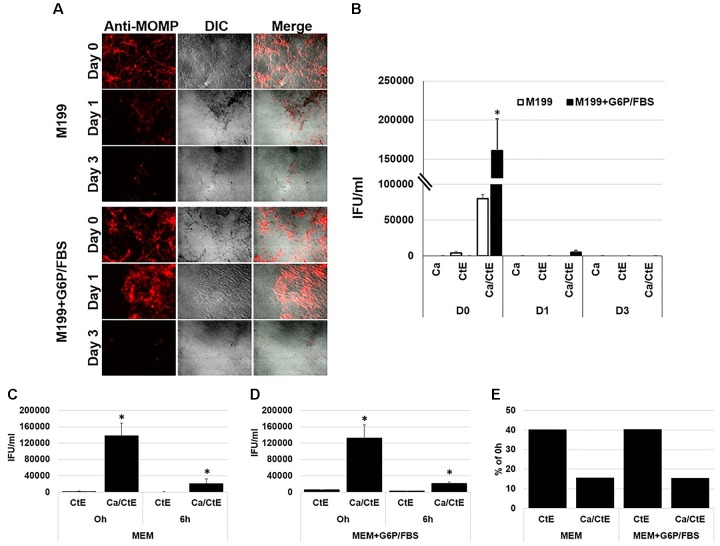
We next wanted to determine if EB bound to Candida remained infectious. To accomplish this, we set up Candida hyphae/EB binding studies as described above in section “Chlamydia EB Bind to the Surface of Candida albicans Yeast and Hyphal Forms” and the methods. Following the incubation period, the cultures were washed and replenished with either M199 or MEM culture medium. M199 is the standard culture medium used for C. albicans culture, whereas MEM is typically used for C. trachomatis culture. We chose to replicate the experiments using both types of media so that no experimental artifacts would be observed due to the use of a medium that is not commonly used for either pathogen. Ca, CtE and Ca/CtE cultures were harvested for IFA and EB titer analysis at 0, 1, and 3 days post incubation (Figures 3A,B). As expected, no inclusions were found in Candida alone samples. On day 0, there were significantly more infectious EB recovered from the Ca/CtE cultures than the CtE alone. These data again confirm EB binding (Figure 3A) to Candida and also indicate that EB remain infectious immediately following binding (Figure 3B). However, by 24h (day 1) post incubation EB bound to Candida are no longer infectious (Figure 3B). In fact, EB bound to Candida lose a significant amount of infectivity within the first 6h of binding (Figures 3C–E). Remarkably, EB bound to Candida lost infectivity at an increased rate compared to EB bound to the coverslip alone. At 6h post binding, 40% of EB incubated in medium alone remained infectious compared to only 16% of EB bound to C. albicans (Figure 3E).
FIGURE 3.
Binding to Candida albicans decreases C. trachomatis EB infectivity. FBS-coated coverslips were inoculated with C. albicans or medium alone and incubated 3h prior to exposure to EB (Ca/CtE or CtE) or 2SPG (Ca). Following incubation of 1h, the cultures were washed, replenished with MEM or M199 medium ± G6P/FBS. Duplicate sets of samples were fixed and processed for IFA or harvested for EB titer analysis at various times post incubation. (A) IFA of Ca/CtE at Day 0, 1, and 3 post incubation in M199 ± G6P/FBS. Chlamydia EB -Red: mouse anti-Chlamydia MOMP/rabbit-anti-mouse Alexa Fluor 594, Candida: DIC 200x magnification. (B) EB titers from Ca, CtE and Ca/CtE cultures at Day 0, 1, and 3 post incubation in M199 ± G6P/FBS. EB titers form CtE and Ca/CtE cultures harvested at 0 and 6h post incubation in MEM (C) or MEM+G6P/FBS (D). (E) Percentage of IFU/ml remaining 6h post incubation compared to 0h for CtE and Ca/CtE cultures in MEM ± G6P/FBS. The data shown represent the means ± SEM of three independent repeats with 3 biological replicates/repeat (n = 9). An asterisk (∗) indicates a significant difference between CtE and Ca/CtE (p ≤ 0.05) as determined by ANOVA and two-sample independent T-tests.
Previous studies have shown that maximal viability of C. trachomatis EB and RB is dependent on availability of specific energy sources. Omsland et al. (2012) demonstrated that EB require glucose-6-phosphate as an energy source. We wanted to ensure that the observed loss of infectivity by Candida bound EB was not simply due to depletion of EB specific energy sources over time. Therefore, we repeated the studies described above using M199 or MEM supplemented with glucose-6-phosphate and 1% FBS (M199 or MEM+G6P/FBS), which has been shown to promote EB viability (Omsland et al., 2012). However, this did not restore or significantly prolong EB infectivity once they were bound to C. albicans hyphal cultures (Figure 3). Control samples were included in these experiments, in which the chlamydial EB were not removed and washed away following the hour adsorption period. The controls were replenished with MEM or MEM+G6P/FBS and examined by titer analysis to determine if the EB remained viable in the medium of the course of the experiment. Data from the control samples indicate that 49–79% of EB remained viable in MEM or MEM+G6P/FBS for 6h in the absence of Candida (Supplementary Figure 3). These data suggest that specific EB/Candida interactions are responsible for the observed decrease in chlamydial infectivity.
Interaction of Chlamydia Elementary Bodies With a Soluble β-Glucan, Laminarin, Inhibits Chlamydial Infectivity in HeLa Cells
Interactions of Chlamydia with the surface of Candida could be mediated via numerous Candida cell wall protein or polysaccharide structures. Mannose polymers, mannans, linked to cell wall proteins, as well as 1,3 and 1,6 linked β-glucans comprise a substantial portion of the Candida cell wall. Furthermore, these structures are found on both yeast and hyphae (Gow et al., 2012; Lowman et al., 2014). Given that we observed EB binding to both of Candida’s morphological forms, these common cell wall structures seemed good candidates for chlamydial binding targets. To examine this possibility, C. trachomatis EB were incubated with soluble fungal cell wall components, glucan-phosphate (β-glucan), a commercial preparation of β-glucans called laminarin, or mannan alone or in combination for 1hr (Williams et al., 1991; Adams et al., 2008; Kruppa et al., 2011). Following exposure to the cell wall components, EB were overlaid onto C. albicans hyphal cultures prepared as described above. The Ca/CtE cultures were fixed and stained for EB visualization by IFA. Binding of EB to Candida was quantified by measuring the fluorescence intensity of chlamydial antibody staining in Ca/CtE cultures prepared with EB exposed to the diluent, mannan, β-glucan or laminarin. Data from these studies indicate that exposure to mannan, β-glucan or laminarin did not significantly block C. trachomatis binding to C. albicans. However, laminarin exposure modestly decreased chlamydial staining intensity, suggesting it may have a partial effect on binding (Figures 4A,B). In similar experiments, HeLa monolayers were infected with C. trachomatis following pre-incubation with diluent, mannan, β-glucan and laminarin alone or in combination. The infected HeLa cultures were harvested for analysis of inclusion development by measuring percent infectivity at 48hpi. Interestingly, pre-incubation of EB with laminarin alone or in combination with β-glucan and mannan significantly decreased chlamydial inclusion formation (Figure 4C, p ≤ 0.01) compared to the diluent control. Additionally, we did not observe EB bound to the surface of infected HeLa cells in these experiments, suggesting that laminarin pre-exposure blocked attachment and entry of EB to the epithelial cells (Supplementary Figure 4). These data indicate that interaction of EB with soluble fungal cell wall carbohydrates does not block EB binding to C. albicans, but these interactions do inhibit chlamydial infectivity.
FIGURE 4.
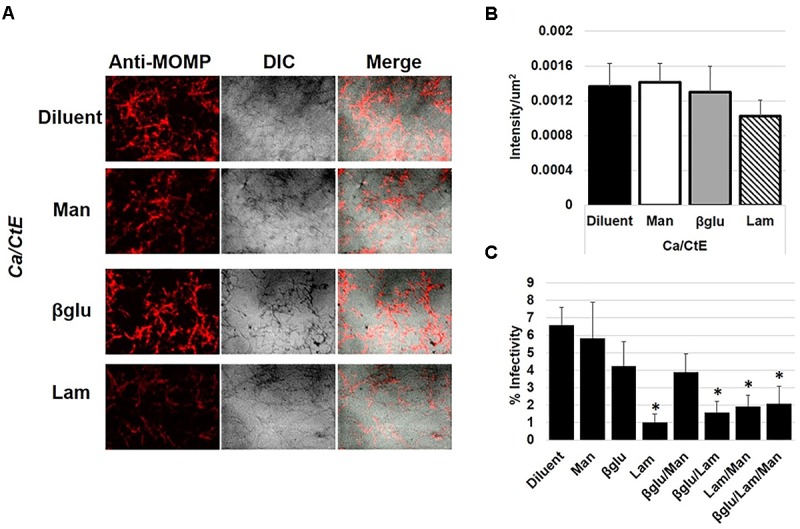
The impact of EB interactions with fungal cell wall components on C. albicans binding and infectivity in HeLa cells. C. trachomatis EB were incubated with diluent (H2O) or fungal cell wall components, mannan (Man), β-glucan (βglu) or laminarin (Lam) for 1h at 37°C prior to incubation with 3h old C. albicans hyphal cultures (A,B) or inoculation of HeLa cell monolayers (C) as described in the methods. (A) Following incubation, Ca/CtE cultures were harvested for IFA. Chlamydia- Red: mouse anti-Chlamydia MOMP/rabbit-anti-mouse Alexa Fluor 594, Candida: DIC 200× magnification. (B) Average intensity/um2 of chlamydia staining represented in panel (A). (C) The percentage of HeLa cells infected with EB exposed to diluent or fungal cell wall component alone or in combination. The data shown represent the means ± SEM of three independent repeats with 3 biological replicates/repeat (n = 9). An asterisk (∗) indicates a significant difference between the experimental sample and the diluent control (p ≤ 0.05) as determined by ANOVA and two-sample independent T-tests.
Discussion
Pathogens do not exist in isolation in the genital tract (Lamont et al., 2011). They interact with members of the microbiome as well as neighboring pathogens. There are numerous examples demonstrating that these interactions can influence pathogenesis to benefit or harm the host or the respective microbes (Peleg et al., 2010; Baquero and Nombela, 2012; Mayer et al., 2013; D’Argenio and Salvatore, 2015; Kong et al., 2015 ). Here we present a novel mechanism of chlamydial inhibition by interactions with a common member of the fungal microbiome and opportunistic pathogen, Candida albicans. We demonstrated that EB bind C. albicans yeast and hyphal morphological forms. Binding to C. albicans significantly decreased C. trachomatis’ ability to infect human cervical epithelial cells. Moreover, we found that interaction of C. trachomatis EB with a soluble preparation of β-glucan, a major component of C. albicans cell walls, diminishes chlamydial inclusion development in vitro. This is an exciting observation, suggesting that direct binding of chlamydial EB to Candida or interaction with shed Candida cell wall components may inhibit C. trachomatis disease progression.
However, studies have demonstrated that Chlamydia-infected women are readily colonized with Candida (Jespers et al., 2014; Van Der Pol et al., 2018). Likewise, Kelly, et al. did not observe an effect on vaginal shedding, or immune response to either organism in mice during concurrent infection (Kelly et al., 2001). These studies suggest that simultaneous Candida colonization may not negatively impact Chlamydia infections in vivo. This could be due to differences in the number and/or location of each organism in the genital tract. For example, vaginal Candida colonization might have less opportunity to influence an established Chlamydia infection in the endometrium. We also observed chlamydial inhibition specifically with laminarin, a soluble preparation of β-glucan. β-glucans are primarily located in the inner layer of the Candida cell wall (Gow et al., 2012). Surface exposure of β-glucans does occur, but it is limited to nanoscopic size areas of the cell wall (Graus et al., 2018). Additionally, chlamydial infections in vivo occur in the presence of seminal fluid, vaginal secretions and additional members of the vaginal microbiota. Semen is known to enhance transmission of human immunodeficiency virus (Lump et al., 2015; Roan and Münch, 2015). Both semen and the vaginal microbiota have been shown to impact the effectiveness of anti-HIV microbicides (Roan and Münch, 2015; Taneva et al., 2018). Thus, it is possible that: 1) there are not sufficient opportunities for direct inhibitory interaction between EB and Candida in vivo to significantly decrease C. trachomatis infection rates; or 2) chemical and/or physical properties of the in vivo environment prevent C. trachomatis inhibition by C. albicans.
Still, we demonstrated that a commercial preparation of β-glucan was sufficient to inhibit Chlamydia trachomatis infection in culture. Laminarin is a soluble form of β-glucan isolated from the algae, Eisenia bicyclis. It contains short 1,3 linked polysaccharides with minimal 1,6 linked branches. Laminarin has been shown to have a wide range of beneficial properties including tumor inhibition, and the ability to act as an antioxidant or anticoagulant (Miao et al., 1995; Ji et al., 2013; Smith et al., 2018). Most notable though, is its ability to stimulate the immune response. β-glucans activate cytokine production via the C-type lectin receptor, Dectin-1 (Smith et al., 2018). Numerous reports indicate that laminarin has either agonistic or antagonistic effects on Dectin-1 activity. The specific preparation used in this study is a Dectin-1 agonist (Smith et al., 2018).
Pre-exposure of C. trachomatis EB to laminarin: (1) modestly decreased Candida/Chlamydia binding, and (2) inhibited inclusion development in HeLa cells. Several possible mechanisms could be responsible for these observations. First, the physical interactions between EB surface molecules and laminarin may block chlamydial adhesins needed for entry into the host cell. Alternatively, laminarin may bind to host cell surface receptor that is required for chlamydial attachment or entry. It is also possible that because laminarin was not removed from the inoculum, inhibition of C. trachomatis may be due to laminarin-induced host cell signaling pathways that negatively impact chlamydial growth. Regardless of the mechanism, laminarin had a strong inhibitory effect on C. trachomatis. This interesting observation opens up the possibility that laminarin could be used as a tool for determining the identity and specific functions of unknown chlamydial adhesins that are required for host cell infection. Others have noted that laminarin possesses antimicrobial properties (Pérez et al., 2016). The results presented here suggest that laminarin may function as an anti-chlamydial compound. Furthermore, β-glucan activation of Dectin-1 is a known mechanism of innate immune training (Cheng et al., 2014; Quintin et al., 2014; van der Meer et al., 2015). Studies have shown that innate immune training by β-glucan/Dectin-1 signaling confers non-specific protective effects against subsequent infections (Cheng et al., 2014; Quintin et al., 2014; van der Meer et al., 2015). Thus, theoretically, a β-glucan-based microbicide would have the potential to elicit an immune response in the genital tract that protects the host from infection with C. trachomatis or other sexually transmitted pathogens. While these possibilities are very intriguing, we must note a limitation of this study. The inhibitory effects of Candida/Chlamydia binding and laminarin pre-exposure were examined using a laboratory strain of Chlamydia trachomatis Serovar E. It is possible that clinical isolates will vary in their interactions with C. albicans and/or laminarin. Future studies are needed to fully investigate the consequences of Chlamydia trachomatis interactions with its fungal neighbor, Candida albicans, and the inhibitory actions of laminarin on chlamydial infections.
Author Contributions
JJ, KK-H, KG, AB, JK, RF, JW, MK, RS, and JH contributed substantially to the design, execution, and/or data collection, and analysis of the experiments within this study. JH drafted the manuscript. All authors contributed to revision and final approval of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful for the excellent work by the Department of Biomedical Sciences Imaging Core Facility staff for sharing their technical expertise. We are especially grateful to Dr. David Williams for sharing reagents with us and for the many helpful conversations regarding this work.
Footnotes
Funding. This work was financially supported by NIH R15AI10985 to MK, NIH/NIAID R15AI117632 and RO3AI128050 to JH, NIH/NIAID R01AI095637 to RS, and NIH/NCRR:1C06RR030651 to East Tennessee State University.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.03270/full#supplementary-material
References
- Adams E. L., Rice P. J., Graves B., Ensley H. E., Yu H., Brown G. D., et al. (2008). Differential high-affinity interaction of dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side-chain branching. J. Pharmacol. Exp. Ther. 325 115–123. 10.1124/jpet.107.133124 [DOI] [PubMed] [Google Scholar]
- Armbruster C. E., Hong W., Pang B., Weimer K. E. D., Juneau R. A., Turner J., et al. (2010). Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling. mBio 1:e00102-10. 10.1128/mBio.00102-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillet A. C., Rehaume L. M., Benham H., O’Meara C. P., Armitage C. W., Ruscher R., et al. (2015). High Chlamydia burden promotes tumor necrosis factor-dependent reactive arthritis in SKG mice. Arthritis Rheumatol. 67 1535–1547. 10.1002/art.39041 [DOI] [PubMed] [Google Scholar]
- Baquero F., Nombela C. (2012). The microbiome as a human organ. Clin. Microbiol. Infect. 18 2–4. 10.1111/j.1469-0691.2012.03916.x [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2017). 2016 Sexually Transmitted Diseases Surveillance. Available at: https://www.cdc.gov/std/stats16/ default.htm [accessed September 24 2018]. [Google Scholar]
- Cheng S.-C., Quintin J., Cramer R. A., Shepardson K. M., Saeed S., Kumar V., et al. (2014). mTOR- and HIF-1 -mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345:1250684. 10.1126/science.1250684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argenio V., Salvatore F. (2015). The role of the gut microbiome in the healthy adult status. Clin. Chim. Acta 451(Pt A), 97–102. 10.1016/j.cca.2015.01.003 [DOI] [PubMed] [Google Scholar]
- Elwell C., Mirrashidi K., Engel J. (2016). Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 14 385–400. 10.1038/nrmicro.2016.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale D., Sandoval B. (1957). Response of mice to the inoculations of both Candida albicans and Escherichia coli. I. The enhancement phenomenon. J. Bacteriol. 73 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow N. A. R., van de Veerdonk F. L., Brown A. J. P., Netea M. G. (2012). Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat. Rev. Microbiol. 10 112–122. 10.1038/nrmicro2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus M. S., Wester M. J., Lowman D. W., Pappas H. C., Lidke K. A., Neumann Correspondence A. K. (2018). Mannan molecular substructures control nanoscale glucan exposure in Candida. Cell Rep. 24 2432–2442.e5. 10.1016/j.celrep.2018.07.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guseva N. V., Dessus-Babus S., Moore C. G., Whittimore J. D., Wyrick P. B. (2007). Differences in Chlamydia trachomatis serovar E growth rate in polarized endometrial and endocervical epithelial cells grown in three-dimensional culture. Infect. Immun. 75 553–564. 10.1128/IAI.01517-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan D. A., Kolter R. (2002). Pseudomonas-Candida interactions: an ecological role for virulence factors. Science 296 2229–2232. 10.1126/science.1070784 [DOI] [PubMed] [Google Scholar]
- Honjo K., van Reekum R., Verhoeff N. P. (2009). Alzheimer’s disease and infection: do infectious agents contribute to progression of Alzheimer’s disease? Alzheimers Dement. 5 348–360. 10.1016/j.jalz.2008.12.001 [DOI] [PubMed] [Google Scholar]
- Jespers V., Crucitti T., Menten J., Verhelst R., Mwaura M., Mandaliya K., et al. (2014). Prevalence and correlates of bacterial vaginosis in different sub-populations of women in sub-Saharan Africa: a cross-sectional study. PLoS One 9:e109670. 10.1371/journal.pone.0109670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C.-F., Ji Y.-B., Meng D.-Y. (2013). Sulfated modification and anti-tumor activity of laminarin. Exp. Ther. Med. 6 1259–1264. 10.3892/etm.2013.1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye D., Levison M. E. (1977). In vitro inhibition of growth of Neisseria gonorrhoeae by genital microorganisms. Sex. Transm. Dis. 4 1–3. [PubMed] [Google Scholar]
- Kelly K. A., Gray H. L., Walker J. C., Rank R. G., Wormley F. L., Fidel P. L. (2001). Chlamydia trachomatis infection does not enhance local cellular immunity against concurrent Candida vaginal infection. Infect. Immun. 69 3451–3454. 10.1128/IAI.69.5.3451-3454.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong E. F., Kucharíková S., Van Dijck P., Peters B. M., Shirtliff M. E., Jabra-Rizk M. A. (2015). Clinical implications of oral candidiasis: host tissue damage and disseminated bacterial disease. Infect. Immun. 83 604–613. 10.1128/IAI.02843-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krezalek M. A., DeFazio J., Zaborina O., Zaborin A., Alverdy J. C. (2016). The Shift of an Intestinal “Microbiome” to a “Pathobiome” governs the course and outcome of sepsis following surgical injury. Shock 45 475–482. 10.1097/SHK.0000000000000534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruppa M., Greene R. R., Noss I., Lowman D. W., Williams D. L. (2011). C. albicans increases cell wall mannoprotein, but not mannan, in response to blood, serum and cultivation at physiological temperature. Glycobiology 21 1173–1180. 10.1093/glycob/cwr051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Campbell L. A. (2003). Chlamydial infections of the cardiovascular system. Front. Biosci. 8 e36–e43. [DOI] [PubMed] [Google Scholar]
- Lamont R., Sobel J., Akins R., Hassan S., Chaiworapongsa T., Kusanovic J., et al. (2011). The vaginal microbiome: new information about genital tract flora using molecular based techniques. BJOG 118 533–549. 10.1111/j.1471-0528.2010.02840.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowman D. W., Greene R. R., Bearden D. W., Kruppa M. D., Pottier M., Monteiro M. A., et al. (2014). Novel structural features in Candida albicans hyphal glucan provide a basis for differential innate immune recognition of hyphae Versus yeast. J. Biol. Chem. 289 3432–3443. 10.1074/jbc.M113.529131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lump E., Castellano L. M., Meier C., Seeliger J., Erwin N., Sperlich B., et al. (2015). A molecular tweezer antagonizes seminal amyloids and HIV infection. eLife 4:e05397. 10.7554/eLife.05397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer F. L., Wilson D., Hube B. (2013). Candida albicans pathogenicity mechanisms. Virulence 4 119–128. 10.4161/viru.22913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullers J. A. (2006). Insights into the interaction between influenza virus and pneumococcus. Clin. Microbiol. Rev. 19 571–582. 10.1128/CMR.00058-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H. Q., Ishai-Michaeli R., Peretz T., Vlodavsky I. (1995). Laminarin sulfate mimics the effects of heparin on smooth muscle cell proliferation and basic fibroblast growth factor-receptor binding and mitogenic activity. J. Cell. Physiol. 164 482–490. 10.1002/jcp.1041640306 [DOI] [PubMed] [Google Scholar]
- Morales D. K., Grahl N., Okegbe C., Dietrich L. E. P., Jacobs N. J., Hogan D. A. (2013). Control of Candida albicans metabolism and biofilm formation by Pseudomonas aeruginosa Phenazines. mBio 4:e00526-12. 10.1128/mBio.00526-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omsland A., Sager J., Nair V., Sturdevant D. E., Hackstadt T. (2012). Developmental stage-specific metabolic and transcriptional activity of Chlamydia trachomatis in an axenic medium. Proc. Natl. Acad. Sci. U.S.A. 109 19781–19785. 10.1073/pnas.1212831109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peipert J. F. (2003). Clinical practice. Genital chlamydial infections. N. Engl. J. Med. 349 2424–2430. [DOI] [PubMed] [Google Scholar]
- Peleg A. Y., Hogan D. A., Mylonakis E. (2010). Medically important bacterial-fungal interactions. Nat. Rev. Microbiol. 8 340–349. 10.1038/nrmicro2313 [DOI] [PubMed] [Google Scholar]
- Peleg A. Y., Tampakakis E., Fuchs B. B., Eliopoulos G. M., Moellering R. C., Mylonakis E. (2008). Prokaryote-eukaryote interactions identified by using Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 105 14585–14590. 10.1073/pnas.0805048105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez M. J., Falqué E., Domínguez H. (2016). Antimicrobial action of compounds from marine seaweed. Mar. Drugs 14:E52. 10.3390/md14030052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters B. M., Ovchinnikova E. S., Krom B. P., Schlecht L. M., Zhou H., Hoyer L. L., et al. (2012). Staphylococcus aureus adherence to Candida albicans hyphae is mediated by the hyphal adhesin Als3p. Microbiology 158(Pt 12), 2975–2986. 10.1099/mic.0.062109-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidwill G. R., Rego S., Jenkinson H. F., Lamont R. J., Nobbs A. H. (2018). Coassociation between group B Streptococcus and Candida albicans promotes interactions with vaginal epithelium. Infect. Immun. 86:e00669-17. 10.1128/IAI.00669-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintin J., Cheng S.-C., van der Meer J. W., Netea M. G. (2014). Innate immune memory: towards a better understanding of host defense mechanisms. Curr. Opin. Immunol. 29 1–7. 10.1016/j.coi.2014.02.006 [DOI] [PubMed] [Google Scholar]
- Roan N. R., Münch J. (2015). Improving preclinical models of HIV microbicide efficacy. Trends Microbiol. 23 445–447. 10.1016/j.tim.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotchford K., Strum A. W., Wilkinson D. (2000). Effect of coinfection with STDs and of STD treatment on HIV shedding in genital-tract secretions: systematic review and data synthesis. Sex. Transm. Dis. 27 243–248. [DOI] [PubMed] [Google Scholar]
- Saniee P., Siavoshi F. (2015). Endocytotic uptake of FITC-labeled anti-H. pylori egg yolk immunoglobulin Y in Candida yeast for detection of intracellular H. pylori. Front. Microbiol. 6:113. 10.3389/fmicb.2015.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K., Kuhlenbäumer G., Rupp J. (2010). Chlamydia pneumoniae infection and Alzheimer’s disease: a connection to remember? Med. Microbiol. Immunol. 199 283–289. 10.1007/s00430-010-0162-1 [DOI] [PubMed] [Google Scholar]
- Smith A. J., Graves B., Child R., Rice P. J., Ma Z., Lowman D. W., et al. (2018). Immunoregulatory activity of the natural product laminarin varies widely as a result of its physical properties. J. Immunol. 200 788–799. 10.4049/jimmunol.1701258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taff H. T., Andes D. R. (2013). Preparation of Candida albicans biofilms for transmission electron microscopy. Bio Protoc. 3:e822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneva E., Sinclair S., Mesquita P. M. M., Weinrick B., Cameron S. A., Cheshenko N., et al. (2018). Vaginal microbiome modulates topical antiretroviral drug pharmacokinetics. JCI Insight 10.1172/jci.insight.99545 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer J. W. M., Joosten L. A. B., Riksen N., Netea M. G. (2015). Trained immunity: a smart way to enhance innate immune defence. Mol. Immunol. 68 40–44. 10.1016/j.molimm.2015.06.019 [DOI] [PubMed] [Google Scholar]
- Van Der Pol B., Daniel G., Kodsi S., Paradis S., Cooper C. K. (2018). Molecular-based testing for sexually transmitted infections using samples previously collected for vaginitis diagnosis. Clin. Infect. Dis. 10.1093/cid/ciy504 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. L., McNamee R. B., Jones E. L., Pretus H. A., Ensley H. E., Browder I. W., et al. (1991). A method for the solubilization of a (1–3)-beta-D-glucan isolated from Saccharomyces cerevisiae. Carbohydr. Res. 219 203–213. [DOI] [PubMed] [Google Scholar]
- Wyrick P. B., Choong J., Knight S. T., Goyeau D., Stuart E. S., MacDonald A. B. (1994). Chlamydia trachomatis antigens on the surface of infected human endometrial epithelial cells. Immunol. Infect. Dis. 4 131–141. [Google Scholar]
- Ziklo N., Huston W. M., Hocking J. S., Timms P. (2016). Chlamydia trachomatis genital tract infections: when host immune response and the microbiome collide. Trends Microbiol. 24 750–765. 10.1016/j.tim.2016.05.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.