Figure 4.
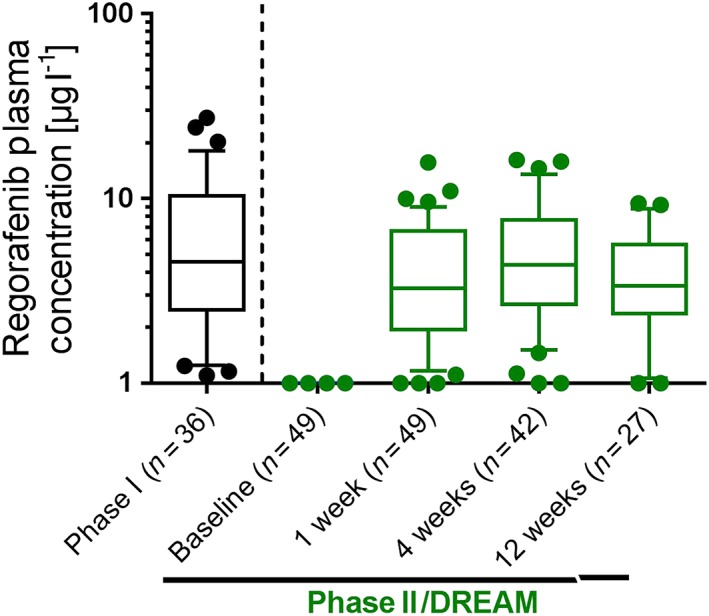
Plasma concentrations of regorafenib after topical administration of 30 mg ml–1, 25 μl three times daily in steady state. There was no significant difference between phase I, in which regorafenib was administered by a study nurse to healthy volunteers, and phase II, when patients self‐administered the eye drops. Data presented as box‐plots (median, boxes 25th and 75th percentile; whiskers 10th and 90th percentile; single values outside 10th and 90th percentile). DREAM, Developing Regorafenib Eye drops for neovascular Age‐related Macular degeneration
