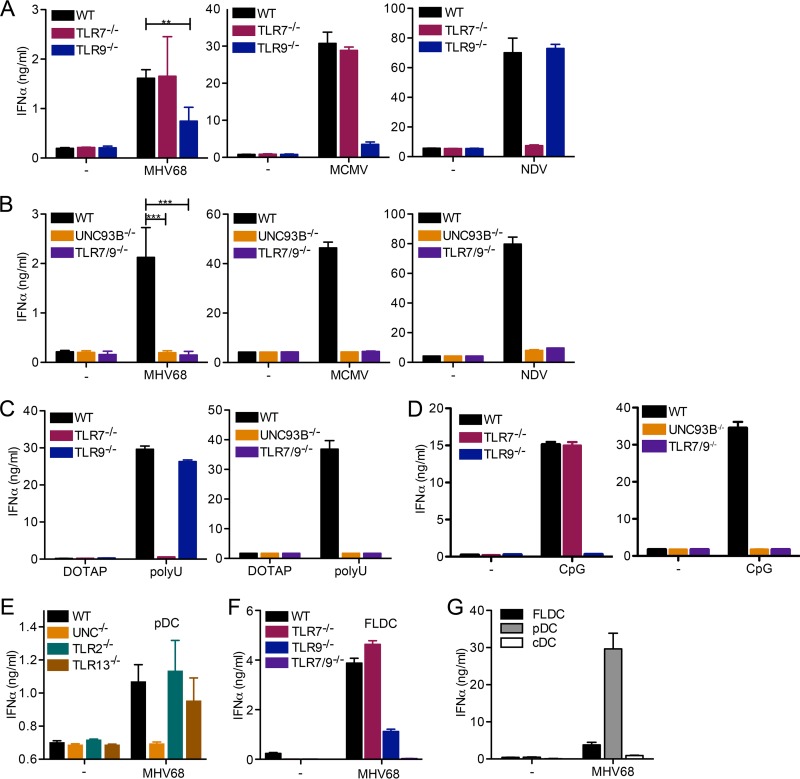
FIG 1.
Both TLR7 and TLR9 are required for the IFN-α response to MHV68 in pDC. (A to D) Bone marrow cells were cultured in the presence of Flt-3 ligand (Flt-3L) for 8 days to produce FLDC, and pDC were MACS purified. WT, Tlr7−/−, and TLR9−/− (A) or WT, Unc93b−/−, or Tlr7−/− Tlr9−/− (B) pDC were mock infected or infected with MHV68, MCMV, or NDV as indicated. (C and D) pDC of the indicated genotype were treated with DOTAP (control) or the TLR7 agonist poly(U) complexed with DOTAP (C) and medium (−) or the TLR9 agonist CpG DNA (D), as indicated. (E) pDC from WT, Unc93b−/−, Tlr2−/−, and Tlr13−/− FLDC were prepared by MACS negative selection. (F) FLDC from WT, Tlr7−/−, Tlr9−/−, and Tlr7−/− Tlr9−/− mice were prepared. (G) WT FLDC were reserved or stained with APC-coupled anti-mouse/human CD45R/B220 and PE-coupled anti-mouse CD11c and then separated into CD45R/B220+ CD11cmid plasmacytoid dendritic cells (pDC) and CD45R/B220− CD11chigh conventional dendritic cells (cDC) by FACS. For panels E to G, equal cell numbers were plated and mock infected or infected with MHV68. For all panels, the IFN-α response was measured by ELISA. In panels A and B, combined duplicates from two independent experiments are shown for MHV68, and log-transformed data for MHV68 were analyzed by a two-tailed unpaired t test (**, P < 0.01; ***, P < 0.001). For all other graphs (MCMV and NDV in panels A and B and C to G), duplicates from one representative experiment are shown.