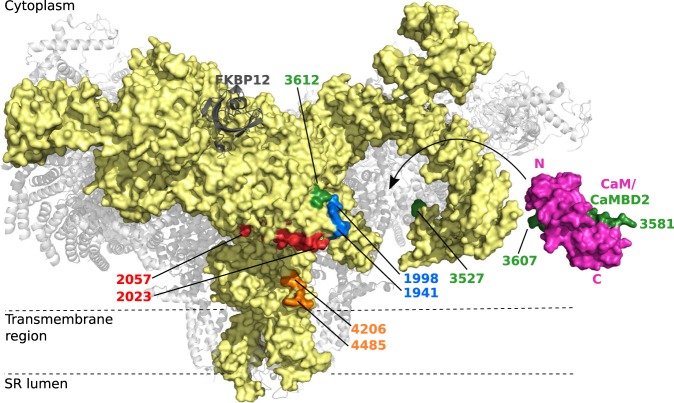
Figure 2. Approximate locations of CaMBDs in the rabbit RyR1 (rRyR1) structure.
Ca2+-saturated CaM (purple) bound to RyR1 CaMBD2 (green) (PDB: 2BCX) was juxtaposed with the 3.8 Å resolution cryo-EM structure of the closed-state rabbit RyR1 homotetramer (PDB: 3J8H) [29,32]. One RyR1 monomer is shown in yellow surface representation, and the other three monomers as gray semi-transparent secondary structure schematics. The CaMBDs are not fully resolved, but the nearest resolved N- and C-terminal amino acids are marked by sequence position and color: CaMBD1a in blue, CaMBD1b in red, CaMBD2 in green, and CaMBD3 in orange. The labeled amino acid positions are numbered according to the equivalent human RyR2 positions.