FIGURE 4:
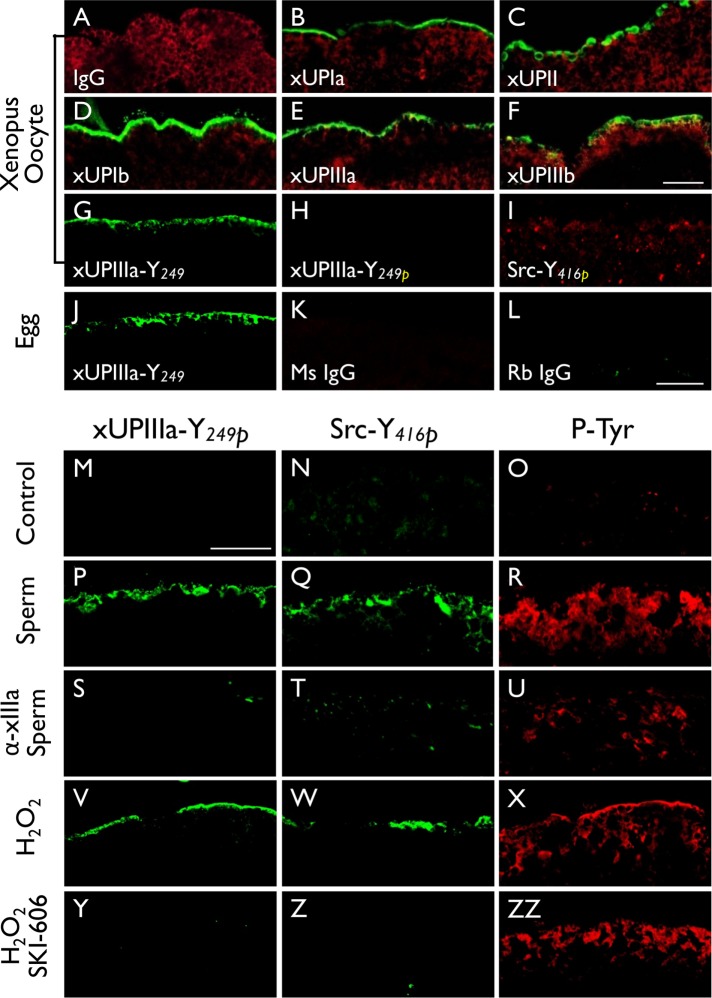
Fertilization of Xenopus laevis (Xl) eggs led to tyrosine phosphorylation of UPIIIa and Src. Xenopus oocytes (A–I) were stained using (A) normal IgG (negative control), or antibodies to (B) Xenopus UPIa or xUPIa (19228), (C) xUPII (13641), (D) xUPIb (13638), (E) xIIIa (19230), (F) xIIIb (4865), (G) xIIIa-Y249 (nonphosphorylated peptide, 35761), (H) xIIIa-Y249P (Tyr-phosphorylated peptide, 35760), or (I) Src-Y416 (Tyr-phosphorylated peptide). Alpha-tubulin was colocalized in A–F as a control. Note the detection of all five Xenopus homologues of mammalian uroplakins on the egg surface. (J–L) Xenopus eggs were stained using antibodies to (J) xIIIa-Y249, (K) normal mouse IgG, or (L) normal rabbit IgG. (M–ZZ) immunofluorescence staining was done using (M–O) control Xenopus eggs, (P–R) sperm-fertilized eggs, (S–U) eggs pretreated with an antibody to xUPIIIa before sperm fertilization, (V–X) hydrogen peroxide-activated eggs, and (Y–ZZ) SKI-606–pretreated eggs before hydrogen peroxide activation. In this series of experiments, each type of egg was stained using antibodies to xIIIa Y249P (first column), Src-Y416P (second), and pan phosphorylated-tyrosine (third). Note the increased Y-phosphorylation of xIIIa and Src in both sperm-fertilized (P, Q) and peroxide-activated (V, W) eggs and the blockage of this reaction by an anti-xUPIIIa antibody (S, T) and Src inhibitor SKI-606 (Y, Z). Bars equal to 20 µm.