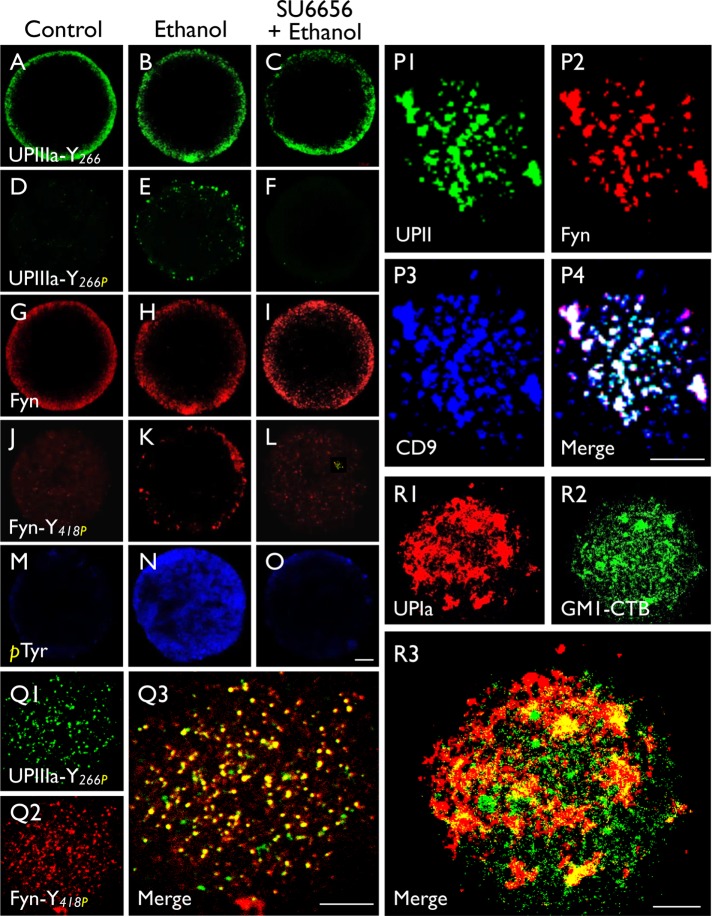
FIGURE 5:
Parthenogenetic activation of mouse eggs led to the tyrosine-phosphorylation of UPIIIa and Fyn. Permeabilized normal eggs (control; first column), ethanol-activated eggs (second column), and SU6656 (Fyn inhibitor)-pretreated and ethanol-activated eggs (third column) were stained using antibodies to (A–C) UPIIIa-Y266 (35759), (D–F) UPIIIa-Y266P (35758) (synthetic peptide containing the phosphorylated (P)-Tyr 266), (G–I) Fyn, (J–L) Fyn-Y418P (synthetic peptide containing the phosphorylated Tyr 418 of Fyn, and (M–O) phosphorylated Tyr. Note in the second column that ethanol-activation led to the Tyr-phosphorylation of UPIIIa-Y266 (E) and Fyn-Y418 (K), and their blockage by SU6656 (third column). (P–R) Immunostaining of intact mouse eggs with antibodies to (P) UPII (S3045)/Fyn/CD9, (Q) UPIIIa-Y266P/Fyn-Y418P, and (R) UPIa/GM1-CTB (a raft marker). Note that in P the substantial colocalization of UPII/Fyn with CD9; in Q the colocalization of tyrosine-phosphorylated UPIIIa and tyrosine-phosphorylated Fyn; and in R the poor colocalization between UPIa (AU-Ia-1) and the raft marker GM-1. Bars equal to 10 µm (A–O) or 5 µm (P–R).