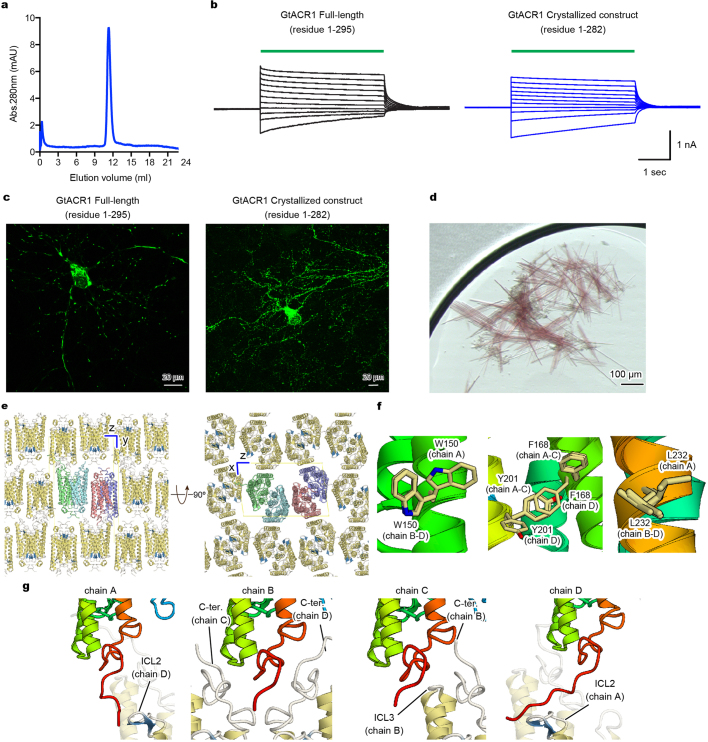
Extended Data Fig. 1. Crystallography.
a, Size exclusion chromatogram of the purified GtACR1 protein used for crystallography. Similar results were seen in more than 20 independent experiments. b, Electrophysiology of full-length GtACR1 (left) and the final crystallization construct (right); whole-cell voltage-clamp recordings in five cells held at −70 mV, with 513 nm light at 1.0 mW mm−2 irradiance delivered with timing as shown with green-coloured bars, while cells were held at resting potentials from −95 mV (lowest trace) to +5 mV (uppermost trace) in steps of 10 mV. Similar results were seen in 3–5 cells from each group, and no significant difference was seen in resting potential, input resistance, reversal potential or photocurrent magnitude. c, Confocal images of cultured hippocampal neurons expressing full-length GtACR1 (left) and the final crystallization construct (right). Similar results were seen in more than five cells from 3–5 coverslips. Note the markedly reduced aggregation of the truncated construct. d, Crystals of GtACR1. Similar crystals were generated in more than 200 experiments. e, Lattice packing of GtACR1 crystals, viewed parallel to the x axis (left) and the y axis (right). f, Different amino acid configurations at different chains within the asymmetric unit of GtACR1. g, C-terminal interactions among different chains within the asymmetric unit of GtACR1.