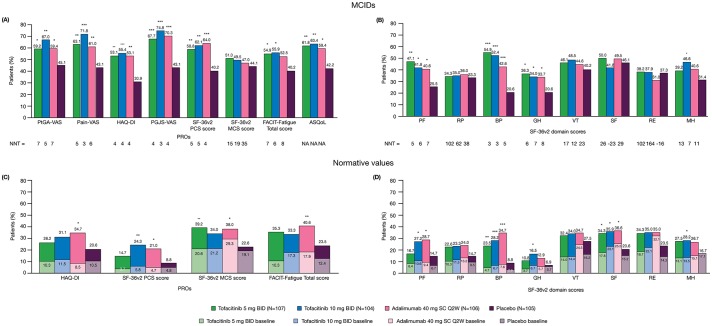
Figure 2.
Percentages of patients reporting improvements from baseline ≥minimum clinically important difference (MCID) and number needed to treat (NNT) in (A) patient-reported outcomes (PROs); (B) Short Form-36 health survey version 2 (SF-36v2) domain scores at month 3 and percentages of patients reporting scores ≥normative values in (C) PROs; (D) SF-36v2 domain scores, at baseline and month 3. *p≤0.05, **p<0.01, ***p<0.001 compared with placebo; p values were based on the normal approximation for the difference in binomial percentages without imputation for missing values (except Health Assessment Questionnaire Disability Index [HAQ-DI] MCID response, which used non-responder imputation); N is the number of patients per group in the Full Analysis Set; the number of patients evaluable for each PRO endpoint may be fewer than N; MCID cut-offs: Patient Global Assessment of disease activity visual analogue scale (PtGA-VAS) decrease from baseline ≥10 mm; Pain-VAS decrease from baseline ≥10 mm; Patient Global Joint and Skin Assessment (PGJS)-VAS decrease from baseline ≥10 mm; SF-36v2: Physical Component Summary (PCS) and Mental Component Summary (MCS) increase from baseline ≥2.5, SF-36v2 domain scores increase from baseline ≥5.0; Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) increase from baseline ≥4.0; Ankylosing Spondylitis Quality of Life (ASQoL) decrease from baseline ≥1.8; HAQ-DI decrease from baseline ≥0.35; MCIDs are defined here in terms of mean change from baseline to month 3 in a treatment group; NNT calculated by the inverse of the percentage of patients receiving active treatments reporting improvements ≥MCIDs minus the percentage of patients in the placebo group reporting improvements ≥MCIDs; the percentage of patients reporting scores ≥normative values: HAQ-DI ≤0.25, FACIT-Fatigue Total score ≥40.1, SF-36v2 each of the component summary and domain scores ≥50. BID, twice daily; BP, bodily pain; GH, general health; MH, mental health; NA, not available; PF, physical functioning; RE, role emotional; RP, role physical; SC Q2W, subcutaneous injection once every 2 weeks; SF, social functioning; VT, vitality.