SUMMARY
Outcomes for diffuse large B-cell lymphoma (DLBCL) in sub-Saharan Africa (SSA) are poorly described. We report mature data from one of the first prospective SSA cohorts. Patients aged ≥18 years with DLBCL were enrolled in Malawi 2013–2017. Participants were treated with CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) chemotherapy and concurrent antiretroviral therapy (ART) if positive for human immunodeficiency virus (HIV+). Eighty-six participants (mean age 47 years, standard deviation 13) were enrolled: 54 (63%) were male and 51 (59%) were HIV+, of whom 34 (67%) were on ART at DLBCL diagnosis. Median CD4 count was 0.113 cells x 109/l (interquartile range [IQR] 0.062–0.227) and 25 (49%) had HIV viral load <400 copies/μl. Participants received median six cycles CHOP (IQR 4–6). No patients were lost to follow-up And the 2-year overall survival was 38% (95% confidence interval 28–49). In multivariable analyses, Eastern Cooperative Oncology Group performance status (PS)≥2 and lactate dehydrogenase (LDH)>2x upper limit of normal (ULN) were associated with mortality. HIV status was not associated with mortality. A simplified prognostic model of LDH>2x ULN and PS≥2 performed at least as well as the age-adjusted International Prognostic Index. DLBCL can be successfully treated in SSA and outcomes did not differ by HIV status. A simplified prognostic model prognosticates well and may be easier to use in resource-limited settings but requires validation.
Keywords: DLBCL, sub-Saharan Africa, HIV, prognosis, chemotherapy
INTRODUCTION
The incidence of non-Hodgkin lymphoma (NHL) is increasing in sub-Saharan Africa (SSA) (Ferlay, et al 2015). Diffuse large B-cell lymphoma (DLBCL) is the commonest NHL subtype in SSA, as in other parts of the world (Naresh, et al 2011). Previous reports from SSA have largely consisted of retrospective studies with non-standardized patient evaluation and treatment, and high loss to follow-up (Bateganya, et al 2011, de Witt, et al 2013, Milligan, et al 2018, Mwanda, et al 2009, Sissolak, et al 2017, Tumwine, et al 2009, Wood, et al 2007).
The standard of care therapy for DLBCL in resource-rich countries is rituximab plus CHOP chemotherapy (cyclophosphamide, doxorubicin, vincristine and prednisone) (Coiffier, et al 2010) and outcomes do not differ between human immunodeficiency virus (HIV)-infected and HIV-uninfected patients in the antiretroviral therapy (ART) era (Besson, et al 2017, Coutinho, et al 2014, Dunleavy, et al 2010, Sparano, et al 2010). However, rituximab remains prohibitively expensive for many low-income countries and its safety has not been tested in settings with high HIV prevalence, although trials are underway (NCT02660710). Therefore, the standard of care throughout SSA remains CHOP without rituximab, but little data exists on the safety and efficacy of this approach, nor on important prognostic factors in this setting.
We previously reported early experience with baseline characteristics and outcomes for all aggressive NHL from the Kamuzu Central Hospital (KCH) Lymphoma Study, a prospective cohort of lymphoproliferative disorders in Malawi (Gopal, et al 2016). Here, we report the more deeply characterized, four-year experience of baseline characteristics, tumour immunophenotypes, treatment course, outcome, and prognostic factors specifically for DLBCL, from the largest and most mature prospective longitudinal cohort in SSA. We hypothesized that CHOP chemotherapy would be safe and effective, and that outcomes would not differ between HIV-infected and HIV-uninfected participants.
METHODS
Study Design and Participants
The KCH Lymphoma Study is a prospective observational cohort of pathologically confirmed lymphoproliferative disorders diagnosed and treated at KCH in Lilongwe, Malawi. KCH is the referral hospital for the central and northern regions of Malawi, with a catchment area of around nine million people. For this report, we included participants ≥18 years of age with newly diagnosed DLBCL or high-grade B-cell NHL, not otherwise specified (B-NHL, NOS), enrolled between June 2013 and May 2017 (Gopal, et al 2016). We excluded participants with DLBCL transformed from prior low-grade lymphoma. All participants were followed until death, loss to follow-up, or administrative censoring on 30 September 2017. The full protocol is described elsewhere (Gopal, et al 2016). Briefly, all diagnoses were pathologically confirmed using tissue biopsy and immunohistochemistry with real-time evaluation in weekly telepathology conferences, involving clinicians and 2–5 pathologists in the United States and Malawi who rendered a consensus opinion. This was followed by confirmation with additional immunophenotyping by both immunohistochemistry and in situ hybridization at the University of North Carolina (UNC) (Montgomery, et al 2016). The Hans algorithm was used to assign germinal centre or non-germinal centre cell-of-origin subtype by immunohistochemistry (Hans, et al 2004).
Written informed consent was obtained from all participants. This study was approved by the UNC Institutional Review Board and Malawi National Health Sciences Research Committee.
Procedures
Patients underwent comprehensive baseline clinical and laboratory evaluation, as described previously (Gopal, et al 2016). Staging was completed by physical examination, chest x-ray, abdominal ultrasound and bone marrow biopsy. Central nervous system sampling and prophylaxis was recommended for those with >1 extranodal site involved and elevated lactate dehydrogenase (LDH), or bone marrow, testicular, epidural, ocular, breast, or paranasal sinus involvement.
CHOP was administered as first-line chemotherapy as described previously (Gopal, et al 2016). All HIV-infected participants were started on, or continued on, ART concurrent with chemotherapy. Relapsed disease was confirmed by biopsy whenever possible. A modified EPIC regimen (etoposide, prednisone, ifosfamide, cisplatin) was typically administered as second-line chemotherapy, as described previously (Kaimila, et al 2017). Neither radiotherapy nor autologous stem cell transplant were typically available.
Statistical Analysis
Differences between HIV-infected and HIV-uninfected patients were assessed using Fisher’s exact tests for categorical data, student’s t-tests for normally distributed data, or Kruskal-Wallis tests for non-normally distributed data. Kaplan-Meier curves were used to estimate overall survival (OS) and progression-free survival (PFS) and the log-rank test was used to assess differences between groups. Cox proportional hazards were used to estimate unadjusted and adjusted hazard ratios for OS. Martingale residuals were evaluated to assess cut-off points for continuous variables in regression analysis. Akaike Information Criterion (AIC) was used to assess model fit in multivariable Cox proportional hazards analysis. All analyses were conducted using Stata version 15.1 (College Station, Texas, USA). A two-sided alpha of 0.05 was considered statistically significant.
RESULTS
Eighty-six participants were identified for the analytic cohort (Table I) [82 (95%) DLBCL, 4 (5%) aggressive B-NHL, NOS]. One participant with DLBCL from transformed low-grade lymphoma was not included. Five participants with DLBCL were excluded as they were enrolled in an ongoing clinical trial of CHOP plus rituximab (clinicaltrials.gov). The mean age at diagnosis was 47 (standard deviation 13) years and 54 (63%) participants were men. Fifty-one participants (59%) were HIV-infected, with HIV diagnosed a median of 2.4 years (interquartile range [IQR] 0.1–6.1) prior to lymphoma diagnosis. Thirty-four (67%) participants were on ART for at least 1 month at the time of DLBCL diagnosis for a median of 3.9 years (IQR 1.3–6.1). Median CD4 count was 0.113 cells x 109/l (interquartile range [IQR] 0.062–0.227), median log viral load was 3.0 (IQR 0–4.6), and 25 (49%) participants had HIV RNA <400 copies/μl at lymphoma diagnosis.
Table I.
Baseline characteristics of patients enrolled in the KCH Lymphoma Study with newly diagnosed diffuse large B cell lymphoma from June 2013 to May 2017.
| n | HIV+ (n=51) |
HIV− (n=35) |
p | |
|---|---|---|---|---|
| Diagnosis, DLBCL, n (%) | 86 | 47 (92) | 35 (100) | 0.14 |
| DLBCL sub-type, germinal centre, n (%) | 65 | 23 (62) | 17 (61) | 1.00 |
| Male, n (%) | 86 | 33 (65) | 21 (60) | 0.82 |
| Age, years, mean (SD) | 86 | 44 (10) | 52 (15) | 0.002 |
| Age >60 years, n (%) | 86 | 3 (6) | 12 (34) | 0.001 |
| District, Lilongwe, n (%) | 84 | 29 (57) | 6 (18) | 0.001 |
| Body mass index, kg/m2, median (IQR) | 84 | 20 (18∣24) | 20 (18∣23) | 0.64 |
| Duration of symptoms <6 months, n (%) |
86 | 40 (78) | 23 (63) | 0.14 |
| B symptoms, n (%) | 86 | 36 (71) | 25 (71) | 1.00 |
| Ann Arbor stage 3 or 4, n (%) | 86 | 30 (59) | 13 (37) | 0.08 |
| ECOG PS ≥2, n (%) | 86 | 20 (39) | 18 (39) | 0.28 |
| LDH >ULN, n (%) | 85 | 45 (90) | 30 (86) | 0.77 |
| LDH >2x ULN, n (%) | 85 | 26 (52) | 11 (31) | 0.08 |
| >1 extranodal site, n (%) | 86 | 8 (16) | 2 (6) | 0.19 |
| International Prognostic Index, n (%) | 85 | 0: 1 (2) 1: 14 (28) 2: 17 (34) 3: 14 (28) 4: 4 (8) 5: 0 (0) |
0: 2 (6) 1: 9 (26) 2: 12 (34) 3: 7 (20) 4: 4 (11) 5: 1 (3) |
0.74 |
| Central nervous system involvement, n (%) |
84 | 4 (8) | 1 (3) | 0.40 |
| Bone marrow involvement, n (%) | 67 | 5 (13) | 0 (0) | 0.06 |
| Splenic involvement, n (%) | 86 | 12 (23) | 10 (28) | 0.62 |
| Liver involvement, n (%) | 86 | 15 (29) | 8 (23) | 0.62 |
| Lymph node largest, cm, median (IQR) | 77 | 8 (5∣11) | 10 (7∣16) | 0.03 |
| Time since HIV diagnosis, years, median (IQR) |
47 | 2.4 (0.1∣6.1) | n/a | n/a |
| ART at diagnosis, n (%) | 51 | 34 (67) | n/a | n/a |
| ART time, years, median (IQR) | 30 | 3.9 (1.3∣6.1) | n/a | n/a |
| CD4 count, cells × 109/l, median (IQR) | 51 | 0.113 (0.062∣0.227) | n/a | n/a |
| HIV viral load, log(10) copies/μl, median (IQR) |
51 | 3.0 (<1.0∣4.6) | n/a | n/a |
| HIV viral load, detectable >400 copies/μl, n (%) |
51 | 26 (51) | n/a | n/a |
| White blood cell count, 109l, median (IQR) |
86 | 6.2 (4.4∣7.9) | 6.3 (4.8∣10) | 0.33 |
| Haemoglobin, g/l, mean (SD) | 86 | 110 (24) | 119 (23) | 0.06 |
| Platelets, 109/l, median (IQR) | 86 | 313 (190∣396) | 328 (228∣441) | 0.42 |
| Creatinine, mg/dL, median (IQR) | 84 | 70.7 (61.9∣88.4) | 61.9 (53.0∣70.7) | 0.03 |
| Estimated glomerular filtration rate, ml/min, median (IQR) (CKD-EPI formula) |
84 | 122 (100∣134) | 121 (110∣136) | 0.49 |
| Bilirubin, µmol/l, median (IQR) | 85 | 5.1 (3.4∣8.5) | 10.3 (5.1∣15.49) | 0.01 |
| Albumin, g/l, mean (SD) | 84 | 33 (7.0) | 35 (6.0) | 0.35 |
ART=anti-retroviral therapy; CKD-EPI= Chronic Kidney Disease Epidemiology Collaboration; DLBCL=diffuse large B cell lymphoma; ECOG PS=Eastern Oncology Cooperative Group performance status; HIV=human immunodeficiency virus; IQR=interquartile range; LDH=lactate dehydrogenase; n/a=not applicable; SD=standard deviation; ULN=upper limit of normal
Compared to the HIV-uninfected group, the HIV-infected group was younger and more likely to come from Lilongwe district. They also had smaller lymph nodes, lower bilirubin and higher creatinine levels.
There were no immunophenotypic differences between HIV-infected and HIV-uninfected groups (Table SI). In the entire cohort, 40/65 (76%) were germinal centre type DLBCL and six (9%) were positive for Epstein–Barr virus (EBV) by in situ hybridisation.
Ten (12%) participants died before they could start CHOP. All other participants were treated with CHOP, with two exceptions. One participant was initially misdiagnosed as Hodgkin lymphoma and treated with four cycles of ABVD (doxorubicin, bleomycin, vinblastine and darcabazine) before the diagnosis was corrected to EBV+-DLBCL of the elderly and treatment was changed to CHOP for an additional four cycles. Another participant was diagnosed with HIV and started ART at the time of DLBCL diagnosis. After excisional biopsy, the patient had no other evidence of disease and has been followed clinically without evidence of recurrence for 11 months at administrative censoring. This may reflect regression of lymphoma after immune reconstitution with ART initiation, as has been previously described (Birendra, et al 2015).
Seventy-four (86%) participants therefore initiated CHOP chemotherapy (Table II) including 42/51 (82%) HIV-infected and 32/35 (94%) HIV-uninfected participants. Participants received a median of 6 cycles of CHOP (IQR 4–6). There was no difference between HIV-infected and HIV-uninfected participants in cumulative chemotherapy dose. Of chemotherapy cycles, 103 (28%) were delayed or reduced for toxicity, 48 (13%) for frailty or old age, 7 (2%) for pharmacy stock-outs and 1 (<1%) for social reasons. Chemotherapy was delayed or reduced more frequently for toxicity in the HIV-infected group and more frequently for age or frailty in the HIV-uninfected group. Grade 3 or 4 neutropenia was more common in the HIV-infected group. There were no differences in other grade 3 or 4 toxicities. There was a total of 14 grade 3/4 non-haematological toxicities, including one grade 4 hypokalaemia, one grade 4 epistaxis, one grade 4 sepsis, two grade 3 emesis, and one grade 3 event of each of the following: hyperglycaemia, upper gastrointestinal haemorrhage, diarrhoea, neuropathy, viral hepatitis, dyspnoea, acute kidney injury, limb oedema and ileus.
Table II.
Treatment characteristics from 74 participants with diffuse large B cell lymphoma treated with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy.
| HIV+ (n=42) | HIV− (n=32) | p | ||
|---|---|---|---|---|
| Cycles, total (n) | 200 | 169 | n/a | |
| Cycles, median (IQR) | 5 (4∣6) | 6 (4∣6) | 0.27 | |
| Days between cycles, median (IQR) |
21 (21∣27) | 21 (21∣23) | 0.10 | |
| Cycles delay ≥ 7 days, n (%) | 30/158 (19) | 16/136 (12) | 0.11 | |
| Chemotherapy delay ≥ 7 days, at least one cycle, n (%) |
23/37 (62) | 13/29 (45) | 0.22 | |
| Dose reduction or delay reason, number of cycles (%) |
Toxicity Age/Frailty Out of stock Social |
72 (36) 17 (8) 3 (2) 1 (<1) |
31 (18) 31 (18) 4 (2) 0 (0) |
<0.01 |
| Doxorubicin dose per cycle, mg/m2, median (IQR) |
42 (38∣50) | 46 (38∣50) | 0.71 | |
| Cyclophosphamide dose per cycle, mg/m2, median (IQR) |
634 (562∣750) | 655 (575∣750) | 0.54 | |
| Intrathecal chemotherapy given, n (%) |
6 (14) | 1 (3) | 0.13 | |
| Neutropenia, grade 3 or 4, number of cycles (%) |
52 (26) | 16 (9) | 0.001 | |
| Neutropenia, grade 3 or 4, at least one cycle, n (%) |
27 (64) | 11 (34) | 0.02 | |
| Anaemia, any grade 3 or 4, n (%) |
5 (12) | 2 (6) | 0.69 | |
| Thrombocytopenia, any grade 3 or 4, n (%) |
0 (0) | 1 (3) | 0.43 | |
| Non-haematological toxicity, any grade 3 or 4, n (%) |
7 (17) | 7 (22) | 0.76 |
HIV=human immunodeficiency virus; IQR=interquartile range
As of 30 September 2017, there were no patients lost to follow-up with a median follow-up of 23.6 months (IQR 16.0–39.9) among patients still alive. Forty-four (59%) participants achieved a complete response (CR) with CHOP, while 12 (16%) achieved a partial response (PR). The response rates did not differ between HIV-infected and HIV-uninfected groups.
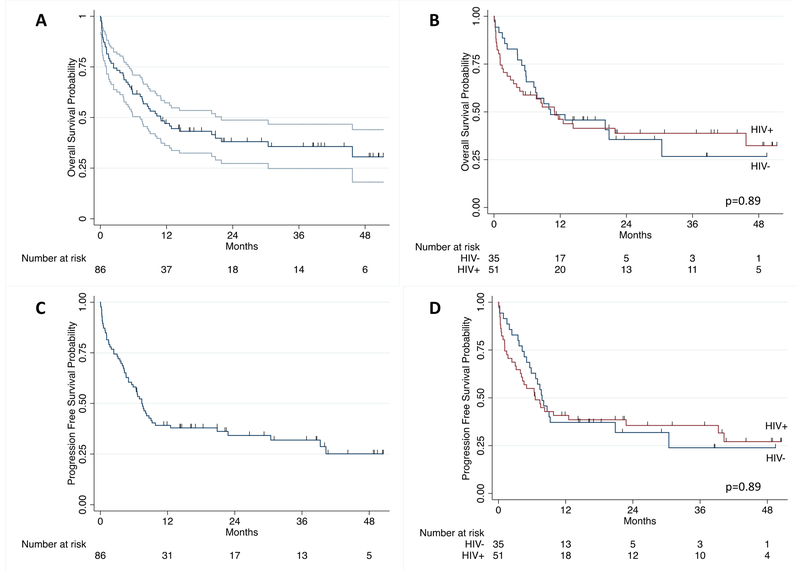
For the entire group, median OS was 10.9 months (95% confidence interval [CI] 7.2–21.9) and median PFS was 7.4 months (5.0–9.8) (Figure 1). One-year OS was 46% (95% CI 35–56) and two-year OS was 38% (28–49). One-year PFS was 38% (28–48) and two-year PFS was 34% (24–44). For patients surviving to CHOP initiation, median OS was 12.9 months (8.7–45.7) with one-year and two-year OS of 52% (40–63) and 42% (30–53), respectively. For those receiving chemotherapy, median PFS was 8.2 months (6.6–22.8) with one-year and two-year PFS of 43% (32–54) and 38% (27–49), respectively. There was no difference in OS or PFS between HIV-infected and HIV-uninfected groups. For those that achieved a CR with CHOP, two-year OS was 66% (95% CI 49–78); for those that achieved a PR with CHOP, two-year OS was 19% (3–46). At the time of censoring, 22 participants had received chemotherapy for relapsed or refractory disease with a median of one additional line of chemotherapy (IQR 1–2), of whom 15 (68%) received EPIC for a median of three cycles (IQR 1–6).
Figure 1. Overall survival and progression-free survival for diffuse large B-cell lymphoma in Malawi.
(A) Overall survival in overall cohort with 95% confidence intervals. (B) Overall survival by human immunodeficiency virus (HIV) status. (C) Progression-free survival in overall cohort with 95% confidence intervals. (D) Progression-free survival by HIV status.
We collected information from family members about circumstances of deaths occurring outside KCH. However, identifying cause of death is often difficult as there is no national death registration in Malawi and patients often die at home or remote facilities with limited diagnostic evaluation. Based on review of all available clinical data and additional information provided by families, central adjudication for cause of death was completed by two study clinicians. There were 13 treatment-related deaths, 36 deaths from DLBCL progression (27 of whom initiated CHOP), and 3 deaths deemed unlikely to be related to CHOP or DLBCL. One treatment-related death occurred in a participant who received only pre-phase cyclophosphamide, vincristine and prednisone, but who did not start CHOP. This death was nevertheless adjudicated as a treatment-related death. Treatment-related deaths occurred largely in patients with adverse baseline DLBCL prognostic factors, especially Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≥2, >1 extranodal site, and higher International Prognostic Index (IPI) (Table SII). Of the 13 treatment-related deaths, six occurred shortly after cycle one of CHOP and were more consistent with tumour lysis or infection, four were later in the course of therapy and consistent with infectious complications, two died within two months of completing chemotherapy with signs and symptoms of heart failure, and one died of epistaxis secondary to thrombocytopenia.
A number of factors were associated with increased mortality in unadjusted Cox proportional hazards analysis including worse ECOG PS, higher stage, >1 extranodal site, higher LDH level, presence of symptoms <6 months, lower body mass index (BMI), splenic involvement, bone marrow involvement, liver involvement, higher white blood cell count, lower haemoglobin, higher platelet count, lower albumin, higher creatinine, and being on ART prior to lymphoma diagnosis (Table III). Of note, only ten participants had a normal LDH level. Analysis of Martingale residuals identified 2.5x the upper limit of normal (ULN) to be the most discriminatory cut-off in our cohort. For simplicity and because the results were similar, we used 2x ULN in subsequent multivariable analysis.
Table III.
Cox proportional hazard univariable and multivariable analysis for overall survival. Adjusted hazard ratio reflects multivariable analysis controlling for all other variables with a value listed.
| Unadjusted hazard ratio |
95 % confidence interval |
p | Adjusted hazard ratio |
95% confidence interval |
p | |
|---|---|---|---|---|---|---|
| HIV infection | 1.04 | 0.60-1.80 | 0.89 | |||
| Germinal centre type DLBCL |
1.01 | 0.52-1.94 | 0.98 | |||
| Male sex | 1.24 | 0.70-2.18 | 0.47 | |||
| Age, per 10 years | 1.03 | 0.85-1.25 | 0.76 | |||
| Age >60 years | 0.66 | 0.31-1.41 | 0.29 | 0.93 | 0.42-2.05 | 0.86 |
| Education, secondary or more |
0.68 | 0.37-1.25 | 0.22 | |||
| Lilongwe resident | 1.31 | 0.75-2.30 | 0.34 | |||
| B symptoms | 1.12 | 0.62-2.05 | 0.70 | |||
| Symptoms <6 months | 2.28 | 1.14-4.56 | 0.02 | |||
| BMI, per kg/m2 | 0.92 | 0.85-0.99 | 0.02 | |||
| Performance status, per ECOG unit |
1.69 | 1.38-2.07 | <0.001 | |||
| Performance status ≥2 | 3.76 | 2.13-6.64 | <0.001 | 2.87 | 1.60-5.13 | <0.001 |
| CNS Involvement | 1.77 | 0.64-4.95 | 0.27 | |||
| Stage, per unit | 1.37 | 1.08-1.75 | 0.01 | |||
| Stage III/IV | 1.95 | 1.12-3.39 | 0.02 | 1.48 | 0.82-2.68 | 0.19 |
| >1 Extranodal sites | 2.15 | 1.00-4.59 | 0.05 | 1.33 | 0.58-3.01 | 0.50 |
| Splenic involvement | 2.09 | 1.17-3.72 | 0.01 | |||
| Liver involvement | 2.07 | 1.17-3.68 | 0.01 | |||
| Bone marrow involvement |
5.84 | 2.20-15.52 | <0.001 | |||
| Bulky disease (largest lymph node ≥10 cm) |
1.05 | 0.61-1.81 | 0.86 | |||
| LDH, per multiple of ULN |
1.08 | 1.03-1.14 | 0.001 | |||
| LDH > ULN | 1.99 | 0.72-5.54 | 0.19 | |||
| LDH >2x ULN | 3.61 | 2.04-6.37 | <0.001 | 2.63 | 1.43-4.84 | 0.002 |
| IPI, per unit | 1.80 | 1.35-2.40 | <0.001 | |||
| White blood cell count, per 109/l |
1.08 | 1.00-1.16 | 0.04 | |||
| Haemoglobin, per g/l | 0.99 | 0.97-1.00 | 0.03 | |||
| Platelet count, per 109/l | 1.02 | 1.00-1.04 | 0.03 | |||
| Albumin, per g/l | 0.93 | 0.89-0.97 | 0.001 | |||
| Creatinine, per 10 µmol/l |
1.11 | 1.02-1.20 | 0.01 | |||
| Glomerular filtration rate, per ml/min |
1.00 | 0.99-1.01 | 0.60 | |||
| Bilirubin, per 10 µmol/l | 1.08 | 1.02-1.15 | 0.62 | |||
| On ART prior to lymphoma diagnosis |
2.35 | 1.01-5.47 | 0.05 | |||
| CD4, per 109 cells/l | 1.08 | 0.17-6.97 | 0.93 | |||
| HIV virus detectable >400 copies/μl |
0.94 | 0.47-1.91 | 0.88 | |||
| HIV viral load, per log(10) copies/μl |
0.97 | 0.82-1.15 | 0.72 | |||
| MYC positive immunohistochemical staining |
1.99 | 0.60-6.54 | 0.26 | |||
| Ki67, per 10% of cells positive |
1.05 | 0.90-1.24 | 0.52 |
BMI=body mass index; CNS=central nervous system; DLBCL=diffuse large B cell lymphoma; ECOG =Eastern Oncology Cooperative Group; HIV=human immunodeficiency virus; IPI=international prognostic index; LDH=lactate dehydrogenase; ULN=upper limit of normal
In a multivariable Cox Proportional Hazards model utilizing standard IPI risk factors, only PS ≥2 [adjusted hazard ratio (AHR) 2.9, 95% CI 1.6–5.2] and LDH >2x ULN (AHR 2.6, 95% CI 1.4–4.8) were associated with increased mortality (Table III).
Exploratory analyses were conducted to identify risk factors that remained significant even after controlling for traditional IPI risk factors. Notably, HIV infection was not associated with mortality in multivariable analysis (AHR 1.0, 95% CI 0.5–1.8; p=0.90). In the entire population, creatinine was associated with an increased hazard for mortality (AHR 1.1, 95% CI 1.0–1.2; per 10 µmol/l; p=0.02) and the presence of B symptoms was associated with a decreased hazard for mortality (AHR 0.4, 95% CI 0.2=0.9; p=0.02). In the subgroup of HIV-infected patients, having previously been on ART at DLBCL diagnosis was associated with worse outcome (AHR 4.8, 95% CI 1.7–14.2, p=0.004), as was a longer duration of HIV infection (AHR 1.1, 95% CI 1.0–1.2 per year; p=0.04). There was a trend toward increased hazard for death with lower HIV viral loads (AHR 0.8, 95% CI 0.7–1.0 per log viral load; p=0.07), but no significant association with CD4 count. In the small subset for whom MYC immunohistochemistry was available (n=26), MYC positive status was associated with increased mortality (AHR 8.1, 95% CI 1.5–44.8; p=0.02). Of note, this exploratory analysis examining additional risk factors for mortality in the multivariable model were consistent using both a baseline model of the standard IPI as well as age-adjusted IPI (aaIPI).
Finally, we sought to assess performance of DLBCL prognostic indices. The IPI, using LDH >2x ULN (AIC 397), out-performed the traditional IPI (AIC 406). The aaIPI, using LDH >2x ULN (AIC 388), outperformed both of these models, as well as the aaIPI with traditional LDH >ULN (AIC 402) (Figure 2). Finally, a simplified model including only PS ≥2 plus LDH >2x ULN performed similarly (AIC 388) to the aaIPI using LDH>2x ULN. There was a suggestion that bone marrow involvement (AHR 2.8, 95% CI 1.1–7.5; p=0.04) or history of ART prior to diagnosis of lymphoma (AHR 2.6, 95% CI 1.1–6.1; p=0.03) could add additional discrimination to the model, but bone marrow staging evaluation was only completed in 67 (78%) participants, and ART status only applied to the HIV-infected population. Adding elevated creatinine to the simplified LDH plus performance status model also improved discrimination (AIC 371) but only six (7%) participants had an elevated creatinine in our cohort. The addition of other risk factors did not further improve these models.
Figure 2. Prognostic models for overall survival of patients with diffuse large B-cell lymphoma in Malawi.
(A) Age-adjusted international prognostic index: sum of LDH>ULN + Ann Arbor stage >2 + ECOG PS ≥2. (B) Age-adjusted international prognostic index using LDH>2x ULN: sum of LDH>2x ULN + Ann Arbor stage >2 + ECOG PS ≥2. (C) Simplified prognostic index of sum of LDH>2x ULN + ECOG PS ≥2.
AIC=Akaike Information Criterion; ECOG PS=Eastern Cooperative Oncology Group performance status; LDH=lactate dehydrogenase, ULN=upper limit of normal.
DISCUSSION
This is the largest and most detailed prospective cohort receiving routine care in SSA for DLBCL, a common curable cancer which is highly associated with HIV. One-year OS was 46% in all participants and 52% in those initiating CHOP chemotherapy, although low compared to resource-rich countries (Barta, et al 2014, Besson, et al 2017, Coutinho, et al 2014, Navarro, et al 2005, Shipp, et al 1993), are similar to or better than estimates from retrospective studies in SSA, which are confounded by selection bias and loss to follow-up (Bateganya, et al 2011, de Witt, et al 2013, Milligan, et al 2018, Mwanda, et al 2009).
The majority of deaths in our cohort were from relapsed or refractory DLBCL rather than treatment. Treatment-related deaths occurred overwhelmingly in those with poor baseline PS and adverse disease characteristics. By traditional aaIPI, only 30% of our cohort presented with an aaIPI of 0 or 1, compared with greater than 50% in a multinational cohort in resource-rich countries (Shipp, et al 1993). Given limited access to advanced imaging, including computed tomography and positron emission tomography, participants are also almost certainly under-staged relative to high-income settings, suggesting disease characteristics are probably somewhat worse for our cohort than measured. While treatment and supportive care must be improved, advanced disease presentations are clearly a driver of suboptimal DLBCL outcomes in Malawi.
We did not identify a difference in outcomes between HIV-infected and HIV-uninfected individuals, similar to most recent reports from resource-rich countries (Baptista, et al 2015, Besson, et al 2017, Coutinho, et al 2014, Navarro, et al 2005). Of interest, being on ART prior to DLBCL diagnosis and longer duration of HIV diagnosis were associated with increased mortality in our cohort, even after adjusting for other important baseline characteristics. A similar observation has been made previously in a large United States HIV-infected cohort (Gopal, et al 2013) and might suggest different DLBCL biology within the HIV-infected population depending on prior ART exposure. These underlying mechanisms may be analogous to demonstrated biological differences for post-transplant lymphoproliferative disorder depending on time after transplant and degree of immunosuppression. Preliminary transcriptome profiling of HIV-infected DLBCL cases from Malawi suggests possible differences in gene expression patterns based on ART status (Fedoriw et al 2017). Additionally, ART initiation probably provides an important anti-DLBCL immunotherapy effect apart from chemotherapy in patients not previously receiving ART (Gopal, et al 2014). Further study is needed to better understand these possible differences in biology and outcomes for HIV-associated DLBCL based on prior ART exposure.
Patients in our cohort present with more advanced disease relative to high-income countries and have limited access to advanced radiographic staging, limiting the utility of traditional prognostic indices derived and validated in resource-rich settings. Specifically, 87% of participants presented with an elevated LDH and therefore elevated LDH >2x ULN performed better as a prognostic marker than simple dichotomization as normal or abnormal. The most predictive models in our cohort were the aaIPI (using LDH >2x ULN) and a simplified model using only ECOG PS ≥2 plus LDH >2x ULN. Given limited advanced imaging in Malawi and throughout SSA, assignment of stage and detection of extranodal sites is probably imprecise, limiting the utility of these baseline prognostic factors. Given the markedly elevated LDH, it is likely that patients in our cohort are understaged compared to similar patients in high income countries. A simplified prognostic model of poor performance status and elevated LDH performs at least as well as the aaIPI and may be more easily implementable in settings similar to Malawi, given the reproducibility and bedside availability of LDH and PS. A simplified prognostic model might also allow for more direct and reliable comparison across cohorts in SSA and other resource-limited settings. We did not have an independent validation cohort to confirm the results of our prognostic modelling, which require replication in other DLBCL populations in SSA.
Strengths of our study include prospective, longitudinal description of pathologically confirmed DLBCL in a part of the world where such data are scarce. Standardization of prospectively defined care with no major eligibility exclusions and administration of standard-dose CHOP as first-line chemotherapy for the vast majority of participants are also major strengths. This makes our study potentially more generalizable than others in SSA, which often describe only participants who survived long enough to receive chemotherapy or with excluded participants for other reasons (Bateganya, et al 2011, de Witt, et al 2013, Mwanda, et al 2009). We had no participants lost to follow-up during the study period utilizing frequent, active mobile phone tracing by staff, limiting overestimation of survival which reliably results from loss to follow-up in SSA (Stanley, et al 2017).
Limitations of this study include possible under-ascertainment of adverse events not detectable by routine laboratories. This study is also limited by being a single institution study with a relatively small sample size, although, to our knowledge, it is one of the largest, well characterized, prospective DLBCL cohorts reported to date from SSA. The lack of death records in the Malawian health system is another limitation, although using detailed clinical data and additional information provided by families, we were able to adjudicate cause of death in nearly all patients through consensus case review. An additional limitation is an absence of cytogenetic data which limits our ability to definitely evaluate for MYC, BCL2 and BCL6 translocations.
To conclude, our study confirms that curative-intent treatment with CHOP for DLBCL is possible in Malawi, in both HIV-infected and HIV-uninfected patients. Deaths in this population are primarily due to relapsed or refractory DBLCL, and treatment-related deaths occurred principally in patients with adverse baseline DLBCL characteristics. A simplified prognostic index of ECOG PS >2 and LDH ≥2 performed at least as well as the traditional IPI and aaIPI in our cohort and may have wide applicability across SSA where advanced radiographic staging is limited, but requires validation. Continued regional efforts to achieve earlier diagnosis in addition to improved treatment and supportive care will hopefully yield continued improvements in cancer survival.
Supplementary Material
Acknowledgments
RESEARCH SUPPORT: This study was funded by NIH Grants # K01TW009488, R21CA180815, U54CA190152, U2GPS001965, and P30CA016086. MSP received support from NIH Research Training Grant # D43TW009340 funded by the NIH Fogarty International Center, NINDS, NIMH, NHBLI and NIEHS and # UM1CA121947, funded by the NIH NCI. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
AUTHORS DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
None of the authors have any have conflicts of interest to disclose.
References
- Baptista MJ, Garcia O, Morgades M, Gonzalez-Barca E, Miralles P, Lopez-Guillermo A, Abella E, Moreno M, Sancho JM, Feliu E, Ribera JM & Navarro JT (2015) HIV-infection impact on clinical-biological features and outcome of diffuse large B-cell lymphoma treated with R-CHOP in the combination antiretroviral therapy era. AIDS, 29, 811–818. [DOI] [PubMed] [Google Scholar]
- Barta SK, Xue X, Wang D, Lee JY, Kaplan LD, Ribera JM, Oriol A, Spina M, Tirelli U, Boue F, Wilson WH, Wyen C, Dunleavy K, Noy A & Sparano JA (2014) A new prognostic score for AIDS-related lymphomas in the rituximab-era. Haematologica, 99, 1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateganya MH, Stanaway J, Brentlinger PE, Magaret AS, Wald A, Orem J & Casper C (2011) Predictors of survival after a diagnosis of non-Hodgkin lymphoma in a resource-limited setting: a retrospective study on the impact of HIV infection and its treatment. Journal of Acquired Immune Deficiency Syndromes, 56, 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson C, Lancar R, Prevot S, Algarte-Genin M, Delobel P, Bonnet F, Meyohas MC, Partisani M, Oberic L, Gabarre J, Goujard C, Boue F, Coppo P, Costello R, Hendel-Chavez H, Mekerri N, Dos Santos G, Recher C, Delarue R, Casasnovas RO, Taoufik Y, Mounier N, Costagliola D & Cohort A-CL (2017) Outcomes for HIV-associated diffuse large B-cell lymphoma in the modern combined antiretroviral therapy era. AIDS, 31, 2493–2501. [DOI] [PubMed] [Google Scholar]
- Birendra KC, Afzal MZ, Wentland KA, Hashmi H, Singh S, Ivan E & Lakhani N (2015) Spontaneous Regression of Refractory Diffuse Large B-Cell Lymphoma with Improvement in Immune Status with ART in a Patient with HIV: A Case Report and Literature Review. Am J Case Rep, 16, 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, Lefort S, Marit G, Macro M, Sebban C, Belhadj K, Bordessoule D, Ferme C & Tilly H (2010) Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood, 116, 2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho R, Pria AD, Gandhi S, Bailey K, Fields P, Cwynarski K, Wilson A, Papanastasopoulos P, Tenant-Flowers M, Webb A, Burns F, Marcus RE, Orkin C, Montoto S & Bower M (2014) HIV status does not impair the outcome of patients diagnosed with diffuse large B-cell lymphoma treated with R-CHOP in the cART era. AIDS, 28, 689–697. [DOI] [PubMed] [Google Scholar]
- de Witt P, Maartens DJ, Uldrick TS & Sissolak G (2013) Treatment outcomes in AIDS-related diffuse large B-cell lymphoma in the setting roll out of combination antiretroviral therapy in South Africa. Journal of Acquired Immune Deficiency Syndromes, 64, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunleavy K, Little RF, Pittaluga S, Grant N, Wayne AS, Carrasquillo JA, Steinberg SM, Yarchoan R, Jaffe ES & Wilson WH (2010) The role of tumor histogenesis, FDG-PET, and short-course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large B-cell lymphoma. Blood, 115, 3017–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoriw Y, Montgomery N, Tomoka T, Dhungel B, Richards K, Selitsky S, Parker J, Gopal S (2017) Identifying transcription and prognostic biomarkers of HIV-associated diffuse large B cell lymphoma from Malawi. In: 16th International Conference on Malignancies in HIV/AIDS. National Cancer Institute, Bethesda, MD page 47, Abstract O21. [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D & Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer, 136, E359–386. [DOI] [PubMed] [Google Scholar]
- Gopal S, Patel MR, Yanik EL, Cole SR, Achenbach CJ, Napravnik S, Burkholder GA, Reid EG, Rodriguez B, Deeks SG, Mayer KH, Moore RD, Kitahata MM, Eron JJ & Richards KL (2013) Temporal trends in presentation and survival for HIV-associated lymphoma in the antiretroviral therapy era. Journal of the National Cancer Institute, 105, 1221–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal S, Patel MR, Achenbach CJ, Yanik EL, Cole SR, Napravnik S, Burkholder GA, Mathews WC, Rodriguez B, Deeks SG, Mayer KH, Moore RD, Kitahata MM, Richards KL & Eron JJ (2014) Lymphoma immune reconstitution inflammatory syndrome in the center for AIDS research network of integrated clinical systems cohort. Clinical Infectious Diseases, 59, 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal S, Fedoriw Y, Kaimila B, Montgomery ND, Kasonkanji E, Moses A, Nyasosela R, Mzumara S, Varela C, Chikasema M, Makwakwa V, Itimu S, Tomoka T, Kamiza S, Dhungel BM, Chimzimu F, Kampani C, Krysiak R, Richards KL, Shea TC & Liomba NG (2016) CHOP Chemotherapy for Aggressive Non-Hodgkin Lymphoma with and without HIV in the Antiretroviral Therapy Era in Malawi. PloS One, 11, e0150445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Mueller-Hermelink H-K, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P, Smith LM, Falini B, Banham AH, Rosenwald A, Staudt LM, Connors JM, Armitage JO & Chan WC (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood, 103, 275–282. [DOI] [PubMed] [Google Scholar]
- Kaimila B, van der Gronde T, Stanley C, Kasonkanji E, Chikasema M, Tewete B, Fox P & Gopal S (2017) Salvage chemotherapy for adults with relapsed or refractory lymphoma in Malawi. Infectious Agents and Cancer, 12, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan MG, Bigger E, Abramson JS, Sohani AR, Zola M, Kayembe MK, Medhin H, Suneja G, Lockman S, Chabner BA & Dryden-Peterson SL (2018) Impact of HIV infection on the clinical presentation and survival of non-Hodgkin lymphoma: a prospective observational study from Botswana. Journal of Global Oncology, 4 2018 April 2 DOI: 10.1200/JGO.17.00084 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery ND, Liomba NG, Kampani C, Krysiak R, Stanley CC, Tomoka T, Kamiza S, Dhungel BM, Gopal S & Fedoriw Y (2016) Accurate Real-Time Diagnosis of Lymphoproliferative Disorders in Malawi Through Clinicopathologic Teleconferences: A Model for Pathology Services in Sub-Saharan Africa. American Journal of Clinical Pathology, 146, 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwanda WO, Orem J, Fu P, Banura C, Kakembo J, Onyango CA, Ness A, Reynolds S, Johnson JL, Subbiah V, Bako J, Wabinga H, Abdallah FK, Meyerson HJ, Whalen CC, Lederman MM, Black J, Ayers LW, Katongole-Mbidde E & Remick SC (2009) Dose-modified oral chemotherapy in the treatment of AIDS-related non-Hodgkin’s lymphoma in East Africa. Journal of Clinical Oncology, 27, 3480–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naresh KN, Raphael M, Ayers L, Hurwitz N, Calbi V, Rogena E, Sayed S, Sherman O, Ibrahim HA, Lazzi S, Mourmouras V, Rince P, Githanga J, Byakika B, Moshi E, Durosinmi M, Olasode BJ, Oluwasola OA, Akang EE, Akenova Y, Adde M, Magrath I & Leoncini L (2011) Lymphomas in sub-Saharan Africa--what can we learn and how can we help in improving diagnosis, managing patients and fostering translational research? British Journal of Haematology, 154, 696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro J-T, Lloveras N, Ribera J-M, Oriol A, Mate J-L & Feliu E (2005) The prognosis of HIV-infected patients with diffuse large B-cell lymphoma treated with chemotherapy and highly active antiretroviral therapy is similar to that of HIV-negative patients receiving chemotherapy. Haematologia, 90, 704–705. [PubMed] [Google Scholar]
- Shipp M, Harrington D, Anderson J, Armitage J, Bonadonna G, Brittinger G, Cabanillas F, Canellos G, Coiffier B, Connors JM, Cowan R, Crowther D, Dahlberg S, Englerhard M, Fisher RI, Gisselbrecht C, Horning SJ, Lister T, Meerwaldt J, Montserrat E, Nissen N, Oken M, Peterson B, Tondini C, Velasquez W & Yeap B (1993) A predictive model for aggressive non-Hodgkin’s lymphoma. New England Journal of Medicine, 329, 987–994. [DOI] [PubMed] [Google Scholar]
- Sissolak G, Seftel M, Uldrick TS, Esterhuizen TM, Mohamed N & Kotze D (2017) Burkitt’s lymphoma and B-cell lymphoma unclassifiable with features intermediate between diffuse large B cell lymphoma and Burkitt’s lymphoma in patients with HIV: outcomes in a South African public hospital. Journal of Global Oncology, 3, 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparano JA, Lee JY, Kaplan LD, Levine AM, Ramos JC, Ambinder RF, Wachsman W, Aboulafia D, Noy A, Henry DH, Von Roenn J, Dezube BJ, Remick SC, Shah MH, Leichman L, Ratner L, Cesarman E, Chadburn A, Mitsuyasu R & Consortium AM (2010) Rituximab plus concurrent infusional EPOCH chemotherapy is highly effective in HIV-associated B-cell non-Hodgkin lymphoma. Blood, 115, 3008–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley CC, Westmoreland KD, Itimu S, Salima A, van der Gronde T, Wasswa P, Mtete I, Butia M, El-Mallawany NK & Gopal S (2017) Quantifying bias in survival estimates resulting from loss to follow-up among children with lymphoma in Malawi. Pediatric Blood & Cancer, 64, e26370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumwine LK, Agostinelli C, Campidelli C, Othieno E, Wabinga H, Righi S, Falini B, Piccaluga PP, Byarugaba W & Pileri SA (2009) Immunohistochemical and other prognostic factors in B cell non Hodgkin lymphoma patients, Kampala, Uganda. BMC Clinical Pathology, 9, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood L, Robinson R, Gavine L, Juritz J & Jacobs P (2007) A single unit lymphoma experience: outcome in a Cape Town academic centre. Transfusion and Apheresis Science, 37, 93–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.