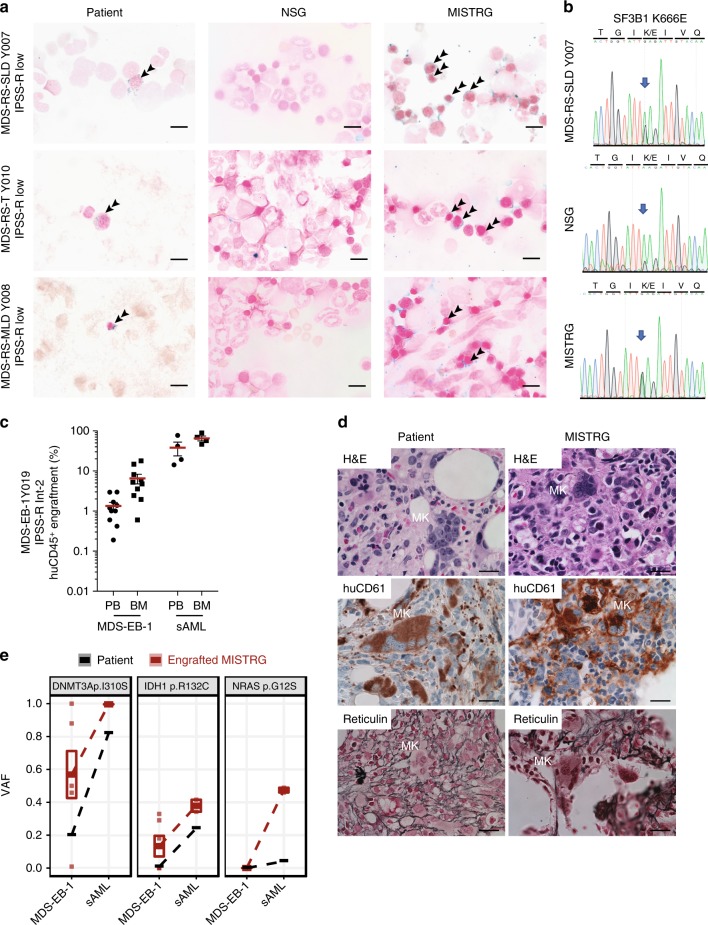
Fig. 4.
MISTRG replicate myelodysplasia and clonal evolution upon disease progression. a NSG and MISTRG were engrafted with low and int-1 risk Sf3B1 mutant myelodysplastic syndrome (MDS) with ring sideroblasts (see Figs. 2, 3) and patient and NSG and MISTRG xenografts were stained with Prussian blue iron stain (scale bars 10 µm, original magnification 60×). b SF3B1 mutation was verified in the patient’s and representative NSG and MISTRG xenografts by Sanger sequencing. c–e MISTRG engrafted with consecutive MDS-EB-1 and secondary acute myeloid leukemia (sAML) samples from the same patient (Y019 and Y028, respectively). c Overall (huCD45+) engraftment in peripheral blood (PB) and bone marrow (BM). Individual mice are represented by symbols, with means ± S.E.M. d Histology from MDS-EB-1 (Y019) diagnostic BM and representative engrafted MISTRG BM. Hematoxylin and eosin (H&E) and huCD61 stains reveal human megakaryocytic dysplasia and reticulin stain reveals bone marrow fibrosis (high-power magnification scale bars 20 µm). e Targeted exome sequencing results from MISTRG xenografted with same patient’s primary MDS-EB-1 diagnosis samples and sAML at the time of disease progression. For each mutation, variant allele frequencies (VAFs) are shown for the patient (black) and representative MISTRG (red) mice with engraftment levels >1%. Mean VAF values between MDS-EB-1 and sAML are connected by lines