Figure 1.
For a Figure360 author presentation of Fig. 1, see the figure legend at https://doi.org/10.1016/j.bpj.2018.11.006.
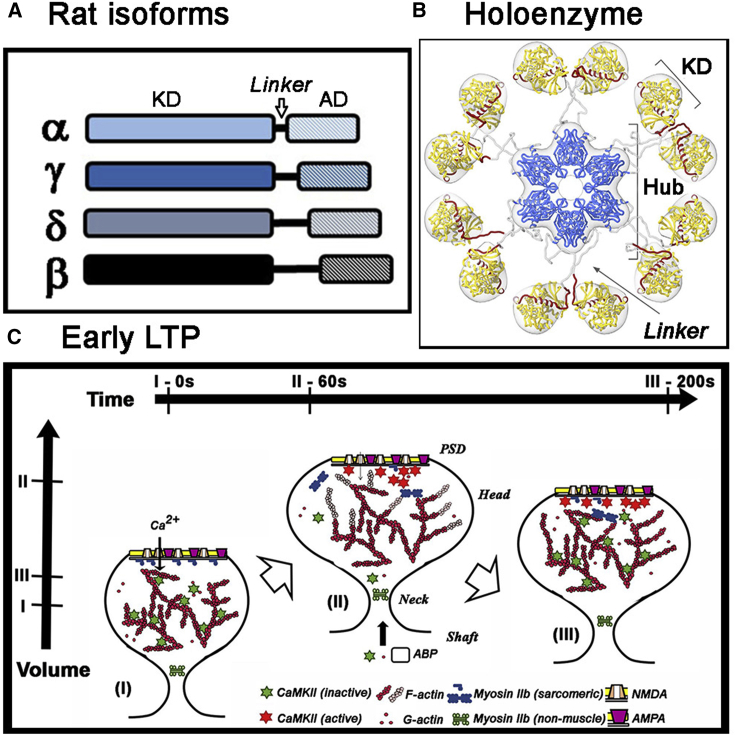
(A) Rat isoforms. Subunits are distinguished by the length and composition of their linkers between the KD and AD. (B) Holoenzyme; Dodecamer with stacked hexamer rings. Linker extensions regulate kinase co-operativity possibly by controlling access of calcium-calmodulin to its R (regulatory segment (red))-binding motif. The dominant extended form of the autoinhibited CaMKII holoenzyme visualized by cryoelectron tomography of the rat α-isoform is shown (with permission from (20)). (C) Stimulus-dependent remodeling of the spine cytoskeleton. Spine morphology is shown in its initial (i) maximally expanded (ii) and stable end states (iii) after synaptic stimulation. (i) to (ii): transient (subsecond) calcium influx triggers calmodulin-mediated CaMKII activation, dissociation from F-actin (red), and sequestration to the PSD. CaMKII kinase activity orchestrates actin polymerization (F-actin; pink) to expand the cytoskeleton. ABP, actin binding protein. Compaction may be powered by PSD-localized myosin miniature-filament formation mediated by MLCK kinase activation. (ii) to (iii): as intracellular calcium returns to basal levels, compaction of the expanded cytoskeleton by myosin is completed and stabilized by the attachment of CaMKII that has entered from the shaft. Horizontal bars denote time stamps for the states, whereas the vertical bars mark relative spine head volumes.