Abstract
OBJECTIVE
Pancreas size is reduced in patients at type 1 diabetes onset and in autoantibody (AAB)-positive donors without diabetes. We sought to determine whether pancreas volume (PV) imaging could improve understanding of the loss of pancreas size in first-degree relatives (FDRs) of patients with type 1 diabetes. We also examined relationships among PV, AAB status, and endocrine and exocrine functions.
RESEARCH DESIGN AND METHODS
We conducted a cross-sectional study that included five groups: AAB− control subjects (no diabetes and no first- or second-degree relatives with type 1 diabetes) (N = 49), AAB− FDRs (N = 61), AAB+ FDRs (N = 67 total: n = 31 with a single positive AAB [AAB+ single] and n = 36 with multiple positive AABs [AAB+ multiple]), and patients with recent-onset type 1 diabetes (<1 year) (N = 52). Fasting subjects underwent 1.5T pancreatic MRI, and PV and relative PV (RPV) (PV-to-BMI ratio) were analyzed between groups and for correlations with HbA1c, C-peptide, glucose, and trypsinogen.
RESULTS
All FDR groups had significantly lower RPV adjusted for BMI (RPVBMI) than control subjects (all P < 0.05). Patients with type 1 diabetes had lower RPVBMI than AAB− FDR (P < 0.0001) and AAB+ multiple (P ≤ 0.013) subjects. Transformed data indicated that trypsinogen levels were lowest in patients with type 1 diabetes.
CONCLUSIONS
This study demonstrates, for the first time, all FDRs having significantly smaller RPVBMI compared with AAB− control subjects. Furthermore, RPVBMI was significantly lower in patients with recent-onset type 1 diabetes than in the AAB− FDR and AAB+ multiple groups. As such, RPVBMI may be a novel noninvasive biomarker for predicting progression through stages of type 1 diabetes risk. This study highlights the potential paracrine relationships between the exocrine and endocrine pancreas in progression to type 1 diabetes in subjects at risk.
Introduction
Despite advances in medical diagnosis and treatment, type 1 diabetes remains one of the costliest diseases in the U.S. in terms of health care dollars spent for diabetes-related costs (1). Immunotherapy-based intervention trials carried out in patients with new-onset type 1 diabetes have demonstrated potential for short-term benefit (e.g., anti-CD20, anti-CD3, and cytotoxic T-lymphocyte–associated antigen 4 Ig) but have failed to demonstrate long-term efficacy (2,3). The inability to provide long-term preservation of β-cell function or prevent type 1 diabetes may stem from unanswered fundamental questions about the pathophysiology of type 1 diabetes in the preclinical stages.
Interest in pancreas size in diabetes has a long history. More than 100 years ago, autopsy studies by Cecil reported reductions in pancreas weight in 25 of 90 patients with diabetes (4). Additional autopsy studies reported variable losses in pancreas weights in patients with diabetes (5–7). As islets comprise only 1–2% of the fractional pancreas volume (PV), the reduction in PV is due to the significant loss of exocrine volume. The loss of pancreas weight has also been corroborated by radiologic imaging studies, including ultrasonography, computed tomography, and MRI, where PV were reduced by 31–52% in patients with long-standing type 1 diabetes and 26–31% in patients with recent-onset type 1 diabetes compared with control subjects (8–13). The reasons for this reduction are unknown and likely occurred months and years before clinical onset. Our previous study suggested that pancreas weight was reduced by 25% in organ donors with a single positive type 1 diabetes–related autoantibody (AAB) (14). This study suggested that reduced pancreatic size may parallel progressive loss of functional β-cell mass. Additional studies are underway to determine whether there is ongoing loss in pancreas size after onset of type 1 diabetes and whether volume loss is ameliorated by exogenous insulin. Studies have also shown a significant but non–clinically meaningful decline in exocrine function (low stool chymotrypsin or elastase) in patients with new-onset type 1 diabetes (15).
Potential explanations for the reduction in pancreas size in type 1 diabetes include loss of insulinotrophic effects on acinar cells and immunologic destruction of exocrine pancreatic tissue (16). Interestingly, pancreatic size is also reduced in fulminant type 1 diabetes, type 2 diabetes, and monogenic diabetes, implicating potentially overlapping mechanisms related to β-cell dysfunction (17–19). Other factors that may impact pancreas size are likely complex interactions between genetics, epigenetics, and environmental agents (20).
Advances in MRI technology continue to improve the quality of pancreas MRI interpretation, and MRI is an indispensable diagnostic tool for pancreatic disease (17,21,22). The purpose of this study was to characterize PV by MRI in first-degree relatives (FDRs) with and without islet-related AABs in comparison with AAB− control subjects with no family history of type 1 diabetes and patients with recent-onset type 1 diabetes. Correlations between PV and biomarkers for endocrine and exocrine functions were also tested.
Research Design and Methods
Subject Enrollment
Control subjects and patients within a year of type 1 diabetes onset (recent onset) were enrolled through the University of Florida Diabetes Institute outpatient clinics. Type 1 diabetes diagnosis was assigned according to American Diabetes Association criteria to patients who were within 1 year of onset (23). The FDR subjects were recruited within the National Institutes of Health (NIH) TrialNet network and were categorized by AAB status (negative, a single AAB, or multiple AABs) (24). Subjects with a history of exocrine insufficiency or pancreatitis were excluded. All studies were performed in accordance with federal guidelines and regulations, and informed consent was provided based on University of Florida institutional review board approval (no. 201600705). Eligible subjects included females and males between the ages of 8 and 50 years who were willing to undergo imaging procedures and phlebotomy. Subjects in all groups were recruited throughout the duration of the study. Notably, our institutional review board approval allowed for inclusion of subjects with diabetes up to 50 years of age, while our analysis plan sought to limit the age range of control subjects and FDRs to those up to 45 years of age in accordance with the upper age limit for antibody screening in the TrialNet Pathway to Prevention Study. Subjects were interviewed for medical history and self-reported race, and body weight and height were measured and recorded. Pregnancy testing was performed in women of childbearing age. BMI was calculated as body weight in kilograms divided by the square of height in meters, and body surface area (Mosteller formula) was calculated as the square root of (height (cm) * body weight (kg)/3,600). Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Florida Clinical and Translational Science Institute (25).
Radiology Protocols
Subjects were given detailed information regarding abdominal imaging without contrast or sedation. After a minimum 4-h fast to enhance differentiation of the pancreas borders, subjects underwent standard abdominal MRI. All MRI exams were performed on a 1.5T Siemens Avanto scanner (Siemens AG, Erlangen, Germany). The examination required a few breath holds and <45 min of exam time. The pancreas MRI protocol included triplanar localizer sequences, an axial nonfat saturated T1-weighted sequence for anatomic imaging and lesion characterization, axial T1-weighted fat saturated and nonfat saturated sequences to differentiate fat from pancreatic lesions, and an axial diffusion-weighted sequence (b500) for lesion detection (21). The parameters of the sequence, notably, the through-plane resolution and the 12-degree flip angle, were modified to increase contrast between the pancreas and surrounding tissues. A modified volumetric interpolated breath-hold examination (VIBE) sequence with a 320 × 240 mm2 in-plane field of view and 3-mm slice thickness was created to provide a voxel resolution of 1.7 × 1.0 × 3.0 mm3. A total of 52 thin, contiguous slices was obtained centered over the region of the pancreas. The PV was calculated by manual delineation of the pancreas from each slice by one of three radiologists specializing in MRI imaging on off-line image-processing workstations (Vitrea FX, Toshiba Medical Systems, Tustin, CA, and Visage PACS, Visage Imaging, San Diego, CA) (Supplementary Fig. 1A and B). The MRI images were reconstructed into three-dimensional sequences providing detailed spatial resolution and greater fat and motion suppression, thereby improving contrast resolution and overall image quality. Interobserver agreement was measured by calculating the intraclass correlation coefficient (ICC) on data collected from three radiologists using five subjects’ MRI scans. The ICC was calculated using a publicly available macro in SAS (26); an ICC value of 1 indicates perfect agreement. PV determinations in this small sample were found to have high agreement (ICC 0.86 [95% CI 0.56, 0.97]).
Laboratory Assays
Whole blood was collected the same day at the University of Florida Clinical and Translational Science Institute Clinical Research Center and shipped overnight on ice packs to Northwest Lipid Metabolism and Diabetes Research Laboratories (Dr. Santica Marcovina, Director; Seattle, WA) for HbA1c analysis. Plasma and serum samples were aliquoted and stored frozen at −20°C until shipment to ARUP Laboratories (University of Utah, Salt Lake City, UT) for serum trypsinogen levels or to Northwest Lipids Research Laboratories for fasting glucose levels and C-peptide analyses.
AAB Assays
Frozen serum aliquots were shipped to the University of Colorado Autoantibody Core (Dr. Liping Yu, Director; Denver, CO) for biochemical AAB analysis. AABs against islet-related autoantigens (i.e., GAD, insulinoma-associated antigen 2, zinc transporter 8, and insulin) were measured using radioimmunoassays as previously described (27).
Statistical Analysis
Data are presented as mean ± SD or 95% CIs with n = number of subjects. All tests were two-sided, and a significance level = 0.05 was used. Statistical analyses were performed using SAS software, version 9.3 (SAS Institute, Cary, NC). Relative PV (RPV) adjusted for BMI (RPVBMI) was determined from PV (mL) divided by BMI. Plots of general variable trends were performed for associations between variables. A linear mixed model was fit to the data to compare mean RPVBMI across the groups, adjusting for observed age and sex differences among the groups while accounting for the nonindependence of data in related individuals, as members of the same family were recruited. Residual variance was allowed to differ across the groups in the model. The Kenward-Roger denominator degrees of freedom was used in making inferences on the differences between the groups. When an overall group comparison was observed, post hoc pairwise comparisons were made with a Tukey correction. A test for a linear trend in RPVBMI across the five groups was also performed. Assumptions of the model (normality, linearity, homoscedasticity) were verified by examination of the studentized residuals. Sample size prevented a comprehensive adjustment for race/ethnicity, but observed differences in racial composition (in the control group) led to two sensitivity analyses: 1) individuals ≤30 years of age (still with adjustment for age and sex) and 2) non-Hispanic white subjects (adjustment for age and sex). Standard linear regression was used to assess the relationship between RPVBMI and duration of type 1 diabetes.
Results
Subject Demographic Data Analyses and MRI Compliance
A total of 246 subjects were recruited. Twelve subjects did not meet inclusion criteria (no subgroup listed, N = 1; no family history of type 1 diabetes, N = 7; and patients with type 1 diabetes aged >45 years (N = 4)). Five subjects did not undergo MRI, which included a family of three whose study visit coincided with scanner maintenance and two boys (10 and 11 years old) with recent-onset type 1 diabetes who declined to undergo the MRI procedure. Two hundred and twenty-nine subjects met inclusion criteria for this study between 2014 and 2017 as follows: control subjects (no diabetes and no first- or second-degree relatives with type 1 diabetes) (n = 49), FDRs with no AABs (AAB− FDR) (n = 61), FDRs with a single positive AAB (AAB+ single) (n = 31), FDRs with multiple positive AABs (AAB+ multiple) (n = 36), and patients with recent-onset type 1 diabetes (n = 52) (Table 1). Overall, 44% of all study subjects were male, and mean ± SD age was 19.6 ± 10.5 years (Table 1). The range of diabetes durations was 0–363 days, with a mean of 175 ± 105 days. Radiologic procedures were well tolerated.
Table 1.
Clinical characteristics of the study group participants
| Overall | Control subjects | AAB− FDR | AAB+ single | AAB+ multiple | Recent onset | |
|---|---|---|---|---|---|---|
| N (%) | 229 | 49 (21.4) | 61 (26.6) | 31 (13.5) | 36 (15.7) | 52 (22.7) |
| Male sex | 101 (44.1) | 20 (40.8) | 23 (37.7) | 9 (29.0) | 22 (61.1) | 27 (51.9) |
| Age (years) | 19.6 ± 10.5 | 18.4 ± 5.9 | 21.1 ± 11.7 | 28.8 ± 13.3 | 15.9 ± 10.7 | 16.2 ± 6.1 |
| Body weight (kg) | 61.0 ± 23.7 | 61.8 ± 17.8 | 63.0 ± 22.1 | 75.3 ± 31.0 | 49.9 ± 22.6 | 61.3 ± 23.0 |
| BMI | 23.2 ± 6.4 | 22.7 ± 4.8 | 23.6 ± 6.1 | 27.6 ± 8.6 | 20.0 ± 5.7 | 22.6 ± 5.9 |
| PV | 74.9 ± 31.8 | 88.2 ± 30.8 | 81.2 ± 29.8 | 89.3 ± 35.4 | 61.2 ± 28.0 | 55.7 ± 21.6 |
| RPVBW | 1.2 ± 0.4 | 1.4 ± 0.4 | 1.3 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.2 | 0.9 ± 0.2 |
| RPVBMI | 3.2 ± 1.1 | 3.9 ± 1.1 | 3.5 ± 0.9 | 3.3 ± 1.1 | 3.0 ± 1.0 | 2.5 ± 0.2 |
| RPV-to-height ratio | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.4 ± 0.1 | 0.3 ± 0.1 |
| Race/ethnicity | ||||||
| NH white | 177 (77.3) | 28 (57.1) | 52 (85.3) | 25 (80.7) | 29 (80.6) | 43 (82.7) |
| NH black | 11 (4.8) | 5 (10.2) | 1 (3.2) | 3 (8.3) | 2 (3.9) | |
| NH Asian | 2 (0.9) | 1 (2.0) | 1 (1.9) | |||
| NH Am. Indian, Alaska Native | — | — | — | — | — | — |
| NH Hawaiian, Pacific Islander | 1 (0.4) | 1 (2.0) | ||||
| Hispanic | 27 (11.8) | 6 (12.2) | 8 (13.1) | 4 (12.9) | 4 (11.1) | 5 (9.6) |
| NH multiple race/unknown | 11 (4.8) | 8 (16.3) | 1 (1.6) | 1 (3.2) | 1 (1.9) | |
| Laboratory tests* | ||||||
| HbA1c (%) | 5.5 ± 1.0 | 5.0 ± 0.3 | 5.1 ± 0.3 | 5.2 ± 0.3 | 5.1 ± 0.4 | 7.0 ± 1.3 |
| HbA1c (mmol/mol) | 36.8 ± 11.2 | 31.5 ± 3.6 | 32.0 ± 3.3 | 33.5 ± 3.5 | 32.6 ± 3.9 | 52.6 ± 13.9 |
| Glucose (mg/dL) | 104.0 ± 37.5 | 90.0 ± 6.0 | 93.0 ± 6.7 | 96.0 ± 8.7 | 94.7 ± 16.0 | 141.4 ± 64.3 |
| C-peptide (ng/mL) | 2.0 ± 1.2 | 2.1 ± 0.7 | 2.3 ± 1.3 | 2.5 ± 1.2 | 1.9 ± 1.2 | 1.2 ± 0.9 |
| Trypsinogen (ng/mL) | 23.9 ± 18.9 | 23.4 ± 10.0 | 25.8 ± 12.4 | 38.2 ± 40.5 | 20.0 ± 8.6 | 16.3 ± 9.9 |
Data are mean ± SD or N (%). Am. Indian, American Indian; NH, non-Hispanic.
*HbA1c (% and mmol/mol), n missing = 5; glucose, n missing = 0; C-peptide, n missing = 1; trypsinogen, exclusion of outlier n = 1 and missing for analysis = 16.
Body Weight, BMI, PV, and RPVBMI by Age
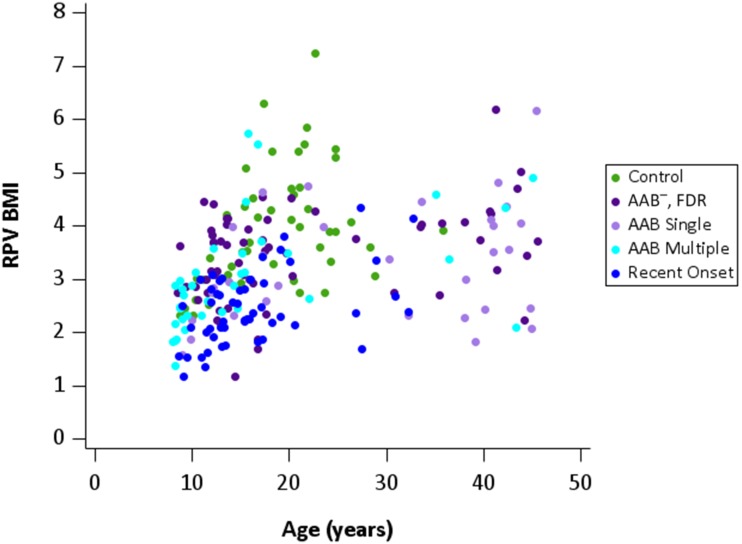
Body weights (kg) and BMI increased with age in all groups, with greater variability of body weight in older subjects compared with those <20 years old (Supplementary Fig. 2A and B). The AAB+ single group had the highest mean age (Table 1). PV showed similar increases with age except for patients with recent-onset type 1 diabetes, who had smaller PV (Supplementary Fig. 3). Both height and weight were significantly (P < 0.05) and independently associated with PV; we thus decided to use BMI in calculating RPVBMI (PV/BMI), as BMI is a standard measure with wide generalizability. RPVBMI showed group trends in comparison with age similar to those for PV in comparison with age. Patients with recent-onset type 1 diabetes had smaller RPVBMI within this age range (Fig. 1 and Table 1). Normalization of PV by body weight (RPVBW [Supplementary Fig. 4]), height, and body surface area mirrored data using the RPVBMI ratio by age (Table 1).
Figure 1.
RPVBMI by age. RPVBMI increased with age to ∼25 years of age in all study groups and then plateaued. Subjects in the group with recent-onset type 1 diabetes had the lowest RPVBMI trends by age.
Reduced RPVBMI in FDRs and Patients With Recent-Onset Type 1 Diabetes
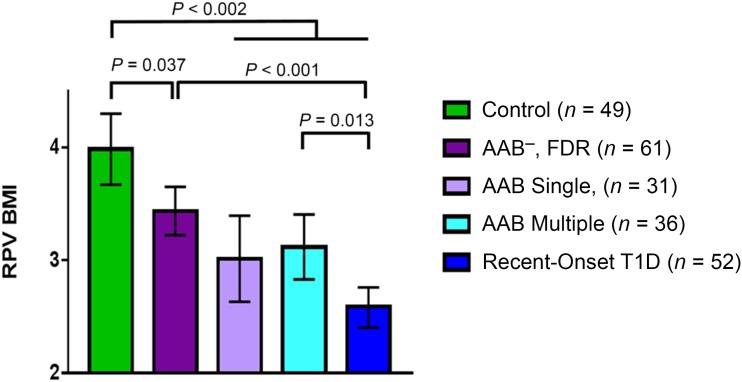
There was a significant linear trend of RPVBMI across groups (P < 0.0001) (Fig. 1 and Supplementary Table 1). Subsequent pairwise testing found that all FDR groups, independent of AAB status, had significantly lower RPVBMI than control subjects. Subjects with recent-onset type 1 diabetes had lower RPVBMI than control, AAB− FDR, and AAB+ multiple subjects (all P ≤ 0.01) (Fig. 2). There was, however, no statistical difference in RPVBMI between ABB+ single subjects and subjects with new-onset type 1 diabetes (P = 0.2651). These results were adjusted for age and sex, given group differences with respect to the distribution of these variables. In addition, we performed two sensitivity analyses: 1) individuals ≤30 years of age (still with adjustment for age and sex) and 2) non-Hispanic white subjects (adjustment for age and sex). While statistical significance was lost for some of the pairwise comparisons, due to a smaller sample size, overall trends of decreasing RPVBMI across groups remained (linear trend test remained statistically significant for both sensitivity analyses).
Figure 2.
Model-estimated mean (95% CI) RPVBMI by study group. Model-estimated mean RPVBMI was significantly different between control subjects and all other groups; a linear trend test was significant. The model adjusted for age and sex and accounted for individuals from the same family. Mean RPVBMI for AAB− FDR subjects was significantly lower than for control subjects and significantly higher than for patients with recent-onset type 1 diabetes (T1D). Mean RPVBMI for AAB+ multiple subjects was significantly different from that for patients with recent-onset type 1 diabetes.
The distribution of PV was also examined with respect to absolute AAB number (i.e., 0, 1, 2, 3, and 4) (Supplementary Fig. 5 and Supplementary Table 2). Although sample sizes in each subgroup were small, in comparison of PV across each of the absolute AAB categories, subjects who had three or four AABs may have had smaller PV compared with that in FDRs with only one AAB as well as in those with two AABs. We also examined whether RPVBMI correlated with diabetes duration in the patients with recent-onset type 1 diabetes. RPVBMI was not linearly associated with duration (R2 = 0.002) (Supplementary Fig. 6).
Endocrine and Exocrine Cell Function Tests
Laboratory assays were complete on the 229 subjects except for five missing samples for HbA1c, one missing sample for C-peptide, and one excluded data point for trypsinogen (Table 1). Patients with recent-onset type 1 diabetes had higher HbA1c and glucose levels and lower C-peptide levels compared with all other groups (Table 1). While the study was not powered to formally test across the study subgroups, we noted decreased trypsinogen levels (though all within reference ranges) in patients with recent-onset type 1 diabetes compared with other groups (P ≤ 0.029) (Supplementary Table 4).
Conclusions
This study showed for the first time, using noninvasive MRI, that AAB− FDR subjects have significantly smaller RPVBMI than AAB− control subjects. The data for AAB+ single subjects also corroborate our previous study that showed reduced pancreas weights in AAB+ single organ donors without diabetes (14). These findings bring renewed interest to the complexity of interactions between the pancreatic endocrine-exocrine compartments in type 1 diabetes and monogenic diabetes (28). They also raise a novel hypothesis that a smaller pancreas in FDRs may act as a predisposing factor for type 1 diabetes risk, particularly if exocrine and endocrine mass reductions occur in parallel. Mechanisms underlying a smaller pancreas are expected to result from complex genetic and environmental interactions that would affect pancreas growth rates and risk of progression to type 1 diabetes. Such interactions could include the in utero environment, childhood, and puberty, during which the majority of rapid organ growth rates occur. Genes modifying type 1 diabetes risk are known from 33-wide sequencing studies, with the HLA class II loci being major determinants of type 1 diabetes development and another ∼40 non-HLA genes with modifying effects (29). HLA class II loci could impact exocrine innate immunity for environmental factors including pathogens such as viruses or bacteria. Future studies examining the potential relationships between HLA and PV are planned. In addition, subclinical exocrine inflammation has been reported in donors with type 1 diabetes and suggests that chronic subclinical pancreatitis could contribute to loss of pancreatic volume (30). Similarly, the subclinical exocrine insufficiency noted in patients with type 1 diabetes may be indicative of a pathogenic loss of exocrine tissue secondary to infection (30–34).
Saisho (35) demonstrated that PV increases with age, with a plateau at ∼20 years of age, and eventually decreases after 60 years of age. Studies have also demonstrated a positive effect of BMI on pancreas size (36). In our study, this positive relationship between age and body or BMI was detected in all groups, with a similar plateau at ∼25 years. Thus, our study confirms numerous previous studies showing that patients with type 1 diabetes have normal body growth rates. However, while the PV also increased with age in control subjects and FDRs, PV and RPVBMI were lowest in patients with recent-onset type 1 diabetes, as found in previous studies (37). In our study, marked reduction in RPVBMI was present at onset of type 1 diabetes. However, RPVBMI was not linearly associated with diabetes duration in our population of patients with type 1 diabetes with duration of 0–363 days. In support of this notion, other cross-sectional studies have reported that PV or pancreas weight did not change with type 1 diabetes duration (38). In children with type 1 diabetes, PV determined by MRI did not correlate with diabetes duration but did correlate positively with total daily dose of insulin (39). Another MRI study in adults with recent-onset type 1 diabetes showed no correlation of PV with diabetes duration, glucose or C-peptide levels, HbA1c, or AAB status (12). These studies contrast with a meta-analysis that was conducted from three studies of patients with type 1 diabetes with a decrease in PV with longest durations, albeit with very high variability between studies (37). Another study was conducted in patients with type 1 diabetes ranging in age from 4 to 67 years, and analysis of MRI and computed tomography images showed a significant loss of RPVBW with age as well as a reduction in RPV with diabetes duration (40). Conflicting findings between studies could be due to methodological differences, including radiology technique and whether the radiographic analysis was performed during fasting or fed conditions. Analysis during fed conditions could interfere with pancreas delineation from adjacent soft tissues, including stomach and small intestines. As with many clinical parameters, individual variability in PV is found, and, thus, longitudinal studies would be particularly informative in a given subject to determine changes relative to baseline. Such data would be informative in developing accurate risk assessments for subjects with stage 1 or stage 2 type 1 diabetes and establishing evaluable responses to interventional therapies in addition to standard end points, such as preservation of C-peptide. It may also be inferred from the results of Regnell et al. (39) that resolution of insulinopenia through insulin therapy stopped further decreases in PV in children with type 1 diabetes. Our observations of decreased RPVBMI in AAB− FDRs with further decreases in AAB+ single and AAB+ multiple subjects suggest to us additional mechanisms leading to loss of PV in addition to subclinical insulinopenia. This notion is further supported by the fact that RPVBMI did not correlate with several measures of β-cell function, including HbA1c, C-peptide levels, and glucose levels, in FDR subjects.
Recent studies indicate that there may be autoimmune infiltration of the exocrine portion of the pancreas, and AABs to exocrine proteins have been reported in patients with type 1 diabetes (20,31,41). Exocrine dysfunction has been reported and indicated by reduction in trypsinogen levels in patients with type 1 diabetes and AAB+ multiple subjects without diabetes (42). While our study was not powered to make formal comparisons across groups, we observed a trend of lower trypsinogen levels in AAB+ multiple subjects and in patients with recent-onset type 1 diabetes, which highlights subclinical exocrine dysfunction in type 1 diabetes. Notably, there was no apparent reduction in trypsinogen among AAB− FDR and AAB+ single versus control subjects despite the apparent reduction in RPVBMI. As reductions in trypsinogen were within typical reference ranges, trypsinogen levels may not be as sensitive as or correlate with RPV measurements in the earliest stages of type 1 diabetes. Whether loss of trypsinogen secretion occurs secondary to decreased insulinotropic effects on the exocrine portion or from a direct autoimmune insult to the acinar cells or a combination of both remains unknown. Further studies would be of interest to elaborate the exocrine autoimmune process in the preclinical phase of type 1 diabetes.
New biomarkers are needed to improve monitoring of progression toward type 1 diabetes. Preventive trials have not provided long-term beneficial outcomes. Heterogeneity during stage 1 and stage 2 type 1 diabetes likely contributes to variable patient responses and may be partly responsible for poor outcomes to date in current clinical trials. Surrogate biomarkers of β-cell function do exist but are relatively insensitive. Imaging studies that can directly assess β-cell mass are still not available for clinical use (43–46). Our study supports the use of abdominal MRI to quantify RPVBMI in subjects at high risk for type 1 diabetes to understand intersubject heterogeneity, changes over time, and association with numbers of AAB. This examination can be readily incorporated with other screening tests while monitoring progression to type 1 diabetes in FDRs. Furthermore, determination of RPVBMI may be valuable in patients after type 1 diabetes onset. For example, patients with smaller RPVBMI may have increased risk for higher glucose variability. Longitudinal studies would be most valuable in extending the current cross-sectional findings but would also be valuable to determine the normal range of RPVBMI variation in a given person over months and years. The rate of decline in RPVBMI may then lead to better prognosis for risk of progression from normoglycemia to dysglycemia and then clinical onset. Additional studies are needed in order to determine whether RPVBMI can be used as a predictive marker in progression to type 1 diabetes. Such studies are expected to confirm the utility of PV-based biomarkers in subjects at high risk and inform the stratification of subjects into future prevention trials.
Supplementary Material
Article Information
Acknowledgments. The authors thank the University of Florida Diabetes Institute; University of California, San Francisco, Diabetes Center; Barbara Davis Center for Diabetes; Endocrinology Specialists/Greenville Health System; All Children’s Hospital; Nemours Children’s Clinic-Orlando; and San Raffaele Hospital outpatient clinic staff who were essential in referring patients for participation in this study, particularly Miriam Citron and Melissa Turnier (University of Florida). The authors are also grateful for the technical support of Elizabeth Hosaka, Joe Canzano, and Lith Nasif (University of Florida). The authors thank Dr. Jonathan Shuster and Dr. Jonathan Williams (University of Florida) for assistance with study design and pancreas imaging.
Funding. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (1DP3-DK-101120-01 to M.L.C.-T. and M.J.H.) and Diabetes Action Research and Education Foundation (00108316 to B.N.). Research reported in this publication was supported by the University of Florida Clinical and Translational Science Institute, which is supported in part by the National Institutes of Health (NIH) National Center for Advancing Translational Sciences under award numbers UL1-TR-000064 and UL1-TR-001427. Some of the subjects in this TrialNet ancillary study were recruited through the Type 1 Diabetes TrialNet Pathway to Prevention Study. The Type 1 Diabetes TrialNet Study Group is a clinical trials network currently funded by the NIH through the NIDDK, the National Institute of Allergy and Infectious Diseases, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, through the cooperative agreements U01-DK-061010, U01-DK-061034, U01-DK-061042, U01-DK-061058, U01-DK-085461, U01-DK-085465, U01-DK-085466, U01-DK-085476, U01-DK-085499, U01-DK-085509, U01-DK-103180, U01-DK-103153, U01-DK-103266, U01-DK-103282, U01-DK-106984, U01-DK-106994, U01-DK-107013, U01-DK-107014, UC4-DK-106993, UC4-DK-117009, and is funded by JDRF International.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.L.C.-T. designed the study, researched data, and wrote the manuscript. S.L.F. and M.J.G. performed statistical calculations, contributed to discussion, and reviewed/edited the manuscript. J.R.G. and R.B. performed PV measurements. B.N. acquired consent from subjects and reviewed the manuscript. All authors approved the final version of the manuscript. M.A.A., D.A.S., and M.J.H. designed the study and reviewed/edited the manuscript. M.L.C.-T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 2018 JDRF nPOD 10th Annual Scientific Meeting, Hollywood, FL, 20–23 February 2018, and at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
Clinical trial reg. no. NCT02234947, clinicaltrials.gov
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-1512/-/DC1.
References
- 1.American Diabetes Association Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018;41:917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Herrath MG, Korsgren O, Atkinson MA. Factors impeding the discovery of an intervention-based treatment for type 1 diabetes. Clin Exp Immunol 2016;183:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staeva TP, Chatenoud L, Insel R, Atkinson MA. Recent lessons learned from prevention and recent-onset type 1 diabetes immunotherapy trials. Diabetes 2013;62:9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cecil RL. A study of the pathological anatomy of the pancreas in ninety cases of diabetes mellitus. J Exp Med 1909;11:266–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 1965;14:619–633 [DOI] [PubMed] [Google Scholar]
- 6.Löhr M, Klöppel G. Residual insulin positivity and pancreatic atrophy in relation to duration of chronic type 1 (insulin-dependent) diabetes mellitus and microangiopathy. Diabetologia 1987;30:757–762 [DOI] [PubMed] [Google Scholar]
- 7.MacLean N, Ogilvie RF. Observations on the pancreatic islet tissue of young diabetic subjects. Diabetes 1959;8:83–91 [DOI] [PubMed] [Google Scholar]
- 8.Fonseca V, Berger LA, Beckett AG, Dandona P. Size of pancreas in diabetes mellitus: a study based on ultrasound. Br Med J (Clin Res Ed) 1985;291:1240–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbeau JP, Poncelet V, Libon E, Derue G, Heller FR. The density, contour, and thickness of the pancreas in diabetics: CT findings in 57 patients. AJR Am J Roentgenol 1992;159:527–531 [DOI] [PubMed] [Google Scholar]
- 10.Altobelli E, Blasetti A, Verrotti A, Di Giandomenico V, Bonomo L, Chiarelli F. Size of pancreas in children and adolescents with type I (insulin-dependent) diabetes. J Clin Ultrasound 1998;26:391–395 [DOI] [PubMed] [Google Scholar]
- 11.Goda K, Sasaki E, Nagata K, Fukai M, Ohsawa N, Hahafusa T. Pancreatic volume in type 1 and type 2 diabetes mellitus. Acta Diabetol 2001;38:145–149 [DOI] [PubMed] [Google Scholar]
- 12.Williams AJ, Thrower SL, Sequeiros IM, et al. . Pancreatic volume is reduced in adult patients with recently diagnosed type 1 diabetes. J Clin Endocrinol Metab 2012;97:E2109–E2113 [DOI] [PubMed] [Google Scholar]
- 13.Gaglia JL, Guimaraes AR, Harisinghani M, et al. . Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. J Clin Invest 2011;121:442–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell-Thompson M, Wasserfall C, Montgomery EL, Atkinson MA, Kaddis JS. Pancreas organ weight in individuals with disease-associated autoantibodies at risk for type 1 diabetes. JAMA 2012;308:2337–2339 [DOI] [PubMed] [Google Scholar]
- 15.Kondrashova A, Nurminen N, Lehtonen J, et al. . Exocrine pancreas function decreases during the progression of the beta-cell damaging process in young prediabetic children. Pediatr Diabetes 2018;19:398–402 [DOI] [PubMed] [Google Scholar]
- 16.Henderson JR, Daniel PM, Fraser PA. The pancreas as a single organ: the influence of the endocrine upon the exocrine part of the gland. Gut 1981;22:158–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haldorsen IS, Ræder H, Vesterhus M, Molven A, Njølstad PR. The role of pancreatic imaging in monogenic diabetes mellitus. Nat Rev Endocrinol 2011;8:148–159 [DOI] [PubMed] [Google Scholar]
- 18.Sasamori H, Fukui T, Hayashi T, et al. . Analysis of pancreatic volume in acute-onset, slowly-progressive and fulminant type 1 diabetes in a Japanese population. J Diabetes Investig 2018;9:1091–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Mrabeh A, Hollingsworth KG, Steven S, Taylor R. Morphology of the pancreas in type 2 diabetes: effect of weight loss with or without normalisation of insulin secretory capacity. Diabetologia 2016;59:1753–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell-Thompson M, Rodriguez-Calvo T, Battaglia M. Abnormalities of the exocrine pancreas in type 1 diabetes. Curr Diab Rep 2015;15:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heyn C, Sue-Chue-Lam D, Jhaveri K, Haider MA. MRI of the pancreas: problem solving tool. J Magn Reson Imaging 2012;36:1037–1051 [DOI] [PubMed] [Google Scholar]
- 22.Di Gialleonardo V, de Vries EF, Di Girolamo M, Quintero AM, Dierckx RA, Signore A Imaging of β-cell mass and insulitis in insulin-dependent (type 1) diabetes mellitus. Endocr Rev 2012;33:892–919 [DOI] [PubMed] [Google Scholar]
- 23.Insel RA, Dunne JL, Atkinson MA, et al. . Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015;38:1964–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Battaglia M, Anderson MS, Buckner JH, et al. . Understanding and preventing type 1 diabetes through the unique working model of TrialNet. Diabetologia 2017;60:2139–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hertzmark E, Spiegelman D. The SAS ICC9 macro [article online], 2010. Available from https://cdn1.sph.harvard.edu/wp-content/uploads/sites/271/2012/09/icc9.pdf. Accessed 10 September 2018
- 27.Bosi E, Boulware DC, Becker DJ, et al.; Type 1 Diabetes TrialNet Study Group . Impact of age and antibody type on progression from single to multiple autoantibodies in type 1 diabetes relatives. J Clin Endocrinol Metab 2017;102:2881–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanyoura M, Jacobsen L, Carmody D, et al. . Pancreatic histopathology of human monogenic diabetes due to causal variants in KCNJ11, HNF1A, GATA6, and LMNA. J Clin Endocrinol Metab 2018;103:35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onengut-Gumuscu S, Chen WM, Burren O, et al.; Type 1 Diabetes Genetics Consortium . Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat Genet 2015;47:381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardt PD, Krauss A, Bretz L, et al. . Pancreatic exocrine function in patients with type 1 and type 2 diabetes mellitus. Acta Diabetol 2000;37:105–110 [DOI] [PubMed] [Google Scholar]
- 31.Nakanishi K, Kobayashi T, Miyashita H, et al. . Relationships among residual beta cells, exocrine pancreas, and islet cell antibodies in insulin-dependent diabetes mellitus. Metabolism 1993;42:196–203 [DOI] [PubMed] [Google Scholar]
- 32.Philippe MF, Benabadji S, Barbot-Trystram L, Vadrot D, Boitard C, Larger E. Pancreatic volume and endocrine and exocrine functions in patients with diabetes. Pancreas 2011;40:359–363 [DOI] [PubMed] [Google Scholar]
- 33.Skog O, Korsgren S, Melhus A, Korsgren O. Revisiting the notion of type 1 diabetes being a T-cell-mediated autoimmune disease. Curr Opin Endocrinol Diabetes Obes 2013;20:118–123 [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Calvo T, Ekwall O, Amirian N, Zapardiel-Gonzalo J, von Herrath MG. Increased immune cell infiltration of the exocrine pancreas: a possible contribution to the pathogenesis of type 1 diabetes. Diabetes 2014;63:3880–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saisho Y. Pancreas volume and fat deposition in diabetes and normal physiology: consideration of the interplay between endocrine and exocrine pancreas. Rev Diabet Stud 2016;13:132–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caglar V, Songur A, Yagmurca M, Acar M, Toktas M, Gonul Y. Age-related volumetric changes in pancreas: a stereological study on computed tomography. Surg Radiol Anat 2012;34:935–941 [DOI] [PubMed] [Google Scholar]
- 37.Garcia TS, Rech TH, Leitão CB. Pancreatic size and fat content in diabetes: a systematic review and meta-analysis of imaging studies. PLoS One 2017;12:e0180911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell-Thompson ML, Kaddis JS, Wasserfall C, et al. . The influence of type 1 diabetes on pancreatic weight. Diabetologia 2016;59:217–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Regnell SE, Peterson P, Trinh L, et al. . Pancreas volume and fat fraction in children with type 1 diabetes. Diabet Med 2016;33:1374–1379 [DOI] [PubMed] [Google Scholar]
- 40.Virostko J, Hilmes M, Eitel K, Moore DJ, Powers AC. Use of the Electronic Medical Record to Assess Pancreas Size in Type 1 Diabetes. PLoS One 2016;11:e0158825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hardt PD, Ewald N, Bröckling K, et al. . Distinct autoantibodies against exocrine pancreatic antigens in European patients with type 1 diabetes mellitus and non-alcoholic chronic pancreatitis. JOP 2008;9:683–689 [PubMed] [Google Scholar]
- 42.Li X, Campbell-Thompson M, Wasserfall CH, et al. . Serum trypsinogen levels in type 1 diabetes. Diabetes Care 2017;40:577–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bini J, Naganawa M, Nabulsi N, et al. . Evaluation of PET brain radioligands for imaging pancreatic β-cell mass: potential utility of 11C-(+)-PHNO. J Nucl Med 2018;59:1249–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eriksson O, Johnström P, Cselenyi Z, et al. . In vivo visualization of β-cells by targeting of GPR44. Diabetes 2018;67:182–192 [DOI] [PubMed] [Google Scholar]
- 45.Balhuizen A, Massa S, Mathijs I, et al. . A nanobody-based tracer targeting DPP6 for non-invasive imaging of human pancreatic endocrine cells. Sci Rep 2017;7:15130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiner T, Thurber G, Gaglia J, et al. . Accurate measurement of pancreatic islet beta-cell mass using a second-generation fluorescent exendin-4 analog. Proc Natl Acad Sci U S A 2011;108:12815–12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.