Abstract
Fibroblast growth factor 21 (FGF21) regulates energy expenditure (EE) and influences weight change during low-protein overfeeding in rodent models. The change in EE after a low-protein overfeeding diet is a predictor of weight change in humans and a feature of the “thrifty” metabolic phenotype. However, there are no studies showing an association between circulating FGF21 and diet-related EE in humans. We assessed the changes in plasma FGF21 concentrations after 24 h of seven dietary interventions with different macronutrient content while in a whole-room indirect calorimeter in 64 healthy subjects with normal glucose regulation. Plasma FGF21 concentration consistently increased by threefold only after the two low-protein (3%) overfeeding diets, one high in carbohydrate (75%) and the other high in fat (46%), with larger increases in FGF21 being associated with greater increases in 24-h EE. Subjects with smaller increases in FGF21 after the low-protein high-fat diet gained more weight after 6 months in free-living conditions. Therefore, the individual predisposition to weight gain over time can be assessed by 24-h overfeeding a low-protein diet and measurements of plasma FGF21 concentrations. Individuals with a blunted FGF21 response to a low-protein diet have a thrifty metabolism and are at risk for future weight gain.
Introduction
As a result of altered energy homeostasis due to the imbalance between energy intake and expenditure (EE), obesity has become more prevalent and a major public health concern. However, the propensity to weight gain is different among individuals, such that some subjects are more resistant to weight gain when overeating because they appear to be more able to dissipate the excess energy than other individuals who instead are more metabolically “thrifty” (1–4). The interindividual diversity in susceptibility to weight gain seems to be secondary to genetic factors and to the capacity to increase EE in response to feeding (i.e., the diet-induced thermogenesis) (3). The manifestation of metabolic phenotypes can be elucidated more clearly when assessing the individual EE response to extreme and macronutrient-unbalanced dietary interventions (2). Specifically, low-protein (<10%) overfeeding has been shown to most effectively uncover the individual propensity to weight gain (3–5), presumably due to the energetic cost required to maintain body lean mass (3,4,6). The underlying hormonal mechanisms by which low-protein overfeeding accentuates interindividual differences in diet-induced thermogenesis and characterizes the subject-specific inclination to weight gain remain unknown.
We previously determined that the acute (24-h) EE response to low-protein overfeeding is a feature of the thrifty/spendthrift metabolic phenotypes, where a smaller increase (or even a decrease) in 24-h EE during this diet predicts weight gain (5). Fibroblast growth factor 21 (FGF21) is a relatively newly identified hormone implicated in the regulation of energy homeostasis (7–9). Rodents who are overfed with a low-protein diet show FGF21-mediated increases in EE compared with a normal-protein diet and are less likely to gain weight (10–13). In humans, sustained low-protein overfeeding increased plasma FGF21 concentrations after 7 (13) or 28 days (10), although no change in EE was observed in the 28-day study (14). The aim of the current study was to determine whether FGF21 concentration changes after 24 h of low-protein overfeeding and to assess whether FGF21 correlates with the diet-induced change in 24-h EE and free-living weight change. We hypothesized that a reduced capacity to respond to a low-protein overfeeding diet by increasing FGF21 concentrations may be a metabolic feature of the “thrifty” metabolic phenotype, indicating a propensity to weight gain.
Research Design and Methods
Subjects
This is an analysis of data from an ongoing study (ClinicalTrials.gov identifier: NCT00523627) aimed to assess whether the 24-h EE responses to fasting and overfeeding predict free-living weight change in healthy, weight-stable individuals (Supplementary Fig. 1). On admission to the clinical research unit, subjects were placed on a standard normal-protein weight-maintaining diet (WMD; 50% carbohydrate [CHO], 30% fat, and 20% protein [Pro]) (15), adjusted daily by the research dietitian to assure weight stability within 1% of admission weight. The average coefficient of variation (CV) of the volunteers’ body weight before the dietary interventions was 0.94 kg. All subjects had normal glucose regulation based on an oral glucose tolerance test (OGTT) performed after 3 days on the WMD (16). Body composition was assessed by DPX-1 (Lunar Corp., Madison, WI) with fat mass and fat free mass calculated from the percentage body fat and weight. After discharge, 48 subjects returned after 6 months (median 6.5 [interquartile range 6.1–7.2] months) to assess weight change. All participants provided written informed consent before beginning the study. The National Institute of Diabetes and Digestive and Kidney Diseases Institutional Review Board approved this study.
Dietary Interventions
The experimental protocol (Supplementary Fig. 2) for dietary manipulation was described previously (17). The assessment of 24-h EE during energy balance was done in two steps. The first eucaloric EE assessment was obtained while subjects resided for 24 h in a whole-room indirect calorimeter and were provided four balanced meals with total daily energy intake calculated using a unit-specific formula to achieve 24-h energy balance in the confined environment of the calorimeter. Secondly, all subjects had another eucaloric EE assessment inside the calorimeter when the total energy intake of four balanced meals was equal to the 24-h EE value calculated during the first eucaloric EE assessment for precise determination of 24-h EE during energy balance.
Subsequently, volunteers had 24-h EE assessments in the calorimeter in random order and separated by a 3-day washout period on the WMD: 24-h fasting, two low-protein (LowPro/HighFat and LowPro/HighCHO), one high-protein, and three normal-protein overfeeding diets with total energy intake determined by doubling the 24-h EE value obtained during energy balance (Table 2 and Supplementary Fig. 3).
Table 2.
Plasma FGF21 concentrations before and after each dietary intervention
| Diet | N | Prediet FGF21 (pg/mL) | Postdiet FGF21 (pg/mL) | Change in FGF21 (pg/mL) | Fold change (ratio) | P value |
|---|---|---|---|---|---|---|
| 24-h fasting | 64 | 124.8 (101.6–153.4) | 82.6 (70.2–97.3) | −65.0 (−87.8 to −42.2) | 0.66 (0.56–0.79) | <0.0001 |
| Energy balance | 64 | 97.6 (76.8–124.1) | 85.5 (68.2–107) | −25.0 (−42.9 to 7.2) | 0.88 (0.75–1.02) | 0.10 |
| Overfeeding | ||||||
| NormalPro/NormalCHO | 63 | 119.3 (90.8–156.7) | 68.3 (52.4–89) | −72.0 (−94.7 to −49.3) | 0.57 (0.47–0.70) | <0.0001 |
| NormalPro/HighCHO | 63 | 123.3 (100.5–151.2) | 99.8 (79–126) | −20.6 (−39.0 to −2.1) | 0.81 (0.72–0.92) | 0.001 |
| NormalPro/HighFat | 63 | 126.2 (102.7–155.2) | 63.7 (50.9–79.6) | −72.5 (−89.0 to −56.0) | 0.50 (0.43–0.59) | <0.0001 |
| HighPro/HighFat | 51 | 125.4 (93.3–165.6) | 31.7 (23.5–42.8) | −121.1 (−148.0 to −94.3) | 0.25 (0.19–0.34) | <0.0001 |
| LowPro/HighFat | 63 | 121.6 (96.1–153.9) | 361.4 (303.2–430.8) | 278.7 (226.5–331.0) | 2.97 (2.54–3.47) | <0.0001 |
| LowPro/HighCHO | 15 | 146.7 (98.1–219.5) | 461.8 (312.8–681.6) | 214.8 (128.6–333.4) | 3.26 (2.34–4.56) | <0.0001 |
Prediet and postdiet plasma FGF21 concentrations are expressed as geometric means with 95% CI. The absolute changes in FGF21 concentrations (pg/mL) are reported as arithmetic means with 95% CI. Fold changes (95% CI) were calculated by exponentiating the average difference between postdiet minus prediet FGF21 concentrations both expressed as log10 values. P values were calculated by paired t test analysis of log10 FGF21 values. Bold values are statistically significant. Macronutrient composition of diets: energy balance diet and NormalPro/NormalCHO overfeeding diet: 50% carbohydrate, 30% fat, 20% protein; NormalPro/HighCHO overfeeding diet: 75% carbohydrate, 5% fat, 20% protein; NormalPro/HighFat overfeeding diet: 20% carbohydrate, 60% fat, 20% protein; HighPro/HighFat overfeeding diet: 26% carbohydrate, 44% fat, 30% protein; LowPro/HighFat overfeeding diet: 51% carbohydrate, 46% fat, 3% protein; and LowPro/HighCHO overfeeding diet: 75% carbohydrate, 22% fat, 3% protein.
Metabolic and Hormone Measurements
The experimental protocol for the assessment of 24-h EE and substrate oxidation inside the whole-room indirect calorimeter was previously described (17,18). VCO2 and VO2 in liters were calculated every minute and extrapolated to the 24-h interval. The 24-h respiratory quotient (RQ) was calculated as the ratio of 24-h VCO2 to 24-h VO2, and 24-h EE was calculated by the Lusk formula (18). Carbohydrate and fat oxidation rates were derived from the 24-h RQ, after accounting for protein oxidation, which was estimated from measurement of 24-h urinary nitrogen excretion (18).
Fasting plasma was collected at entry and at exit from the calorimeter in EDTA-containing tubes and frozen to −70°C for later measurements. FGF21 concentrations were measured by ELISA (R&D Systems, Minneapolis, MN). Intraassay and interassay CVs were 2.5% and 5.2%, respectively.
Statistics
Nonnormally distributed FGF21 concentrations were analyzed as log10 values, and results were presented as the geometric mean with 95% CI. The change in FGF21 concentration after each diet was assessed by paired t test. For each subject, all of the fasting FGF21 measurements obtained before entering the calorimeter were averaged and used in ANOVA to determine differences according to sex and ethnicity and in correlation analysis with anthropometric characteristics.
Results
Baseline characteristics of the study cohort are presented in Table 1. The fasting FGF21 concentration correlated with anthropometric characteristics (Supplementary Fig. 4) and differed by ethnicity, such that, on average, FGF21 was lower by 60% (CI 48–69; P < 0.0001) in blacks compared with other ethnicities.
Table 1.
Baseline characteristics of the study group
| All subjects (N = 64) | Women (n = 12) | Men (n = 52) | |
|---|---|---|---|
| Age (years) | 37 ± 10 (18, 54) | 33 ± 8 (20, 45) | 38 ± 10 (18, 54) |
| Ethnicity (n) | |||
| Black | 14 | 5 | 9 |
| White | 19 | 4 | 15 |
| Hispanic | 11 | 1 | 10 |
| Native American | 20 | 2 | 18 |
| Body weight (kg) | 78.5 ± 12.2 (47.5, 107.8) | 74.4 ± 16.8 (47.5, 107.8) | 79 ± 11 (56, 105) |
| BMI (kg/m2) | 26.2 ± 3.9 (17.8, 39.1) | 26.9 ± 5.8 (17.8, 39.1) | 26 ± 3 (18, 37) |
| Body fat (%) | 27.6 ± 10.0 (6.9, 53.8) | 40.4 ± 8.4 (24.2, 53.8) | 25 ± 8 (6.9, 38)* |
| FM (kg) | 22.1 ± 10.0 (4.9, 56.9) | 31.2 ± 13.0 (13.6, 56.9) | 20 ± 8 (4.9, 36)* |
| FFM (kg) | 56.4 ± 9.3 (33.9, 79.4) | 43.2 ± 4.9 (33.9, 50.9) | 59 ± 7 (47, 79.4)* |
| 24-h EE (kcal/day) | 2,038 ± 283 (1,502, 2,810) | 1,802 ± 223 (1,502, 2,290) | 2,094 ± 268 (1,573, 2,810)* |
| 24-h RQ (ratio) | 0.87 ± 0.03 (0.80, 0.93) | 0.86 ± 0.03 (0.81, 0.91) | 0.87 ± 0.03 (0.80, 0.93) |
| Fasting glucose (mg/dL) | 92.0 ± 5.07 (80.0, 99.0) | 91.0 ± 3.3 (86.5, 97.0) | 92.3 ± 5.4 (80, 99) |
| 2-h OGTT glucose (mg/dL) | 103.8 ± 19.9 (65, 138) | 104.2 ± 16.5 (80, 130) | 103.7 ± 20.8 (65, 138) |
| Fasting plasma FGF21 (pg/mL) | 128.8 (105.8–156.9) [13.0, 492.9] | 119.0 (70.6–200.5) [22.4, 288.2] | 131.2 (105.4–163.4) [13.0, 492.9] |
Unless otherwise stated, data are presented as mean ± SD (minimum, maximum), except for FGF21 where values are presented as geometric mean with its 95% CI [minimum, maximum]. Fasting plasma FGF21 concentration was calculated as the average of all prediet fasting measurements. FFM, fat free mass; FM, fat mass.
*P < 0.05 vs. women, calculated by Student t test.
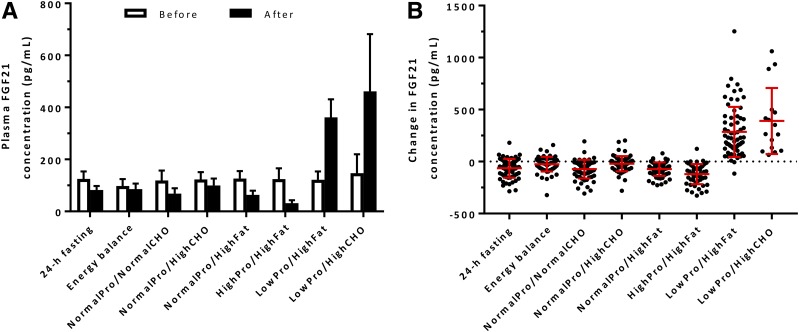
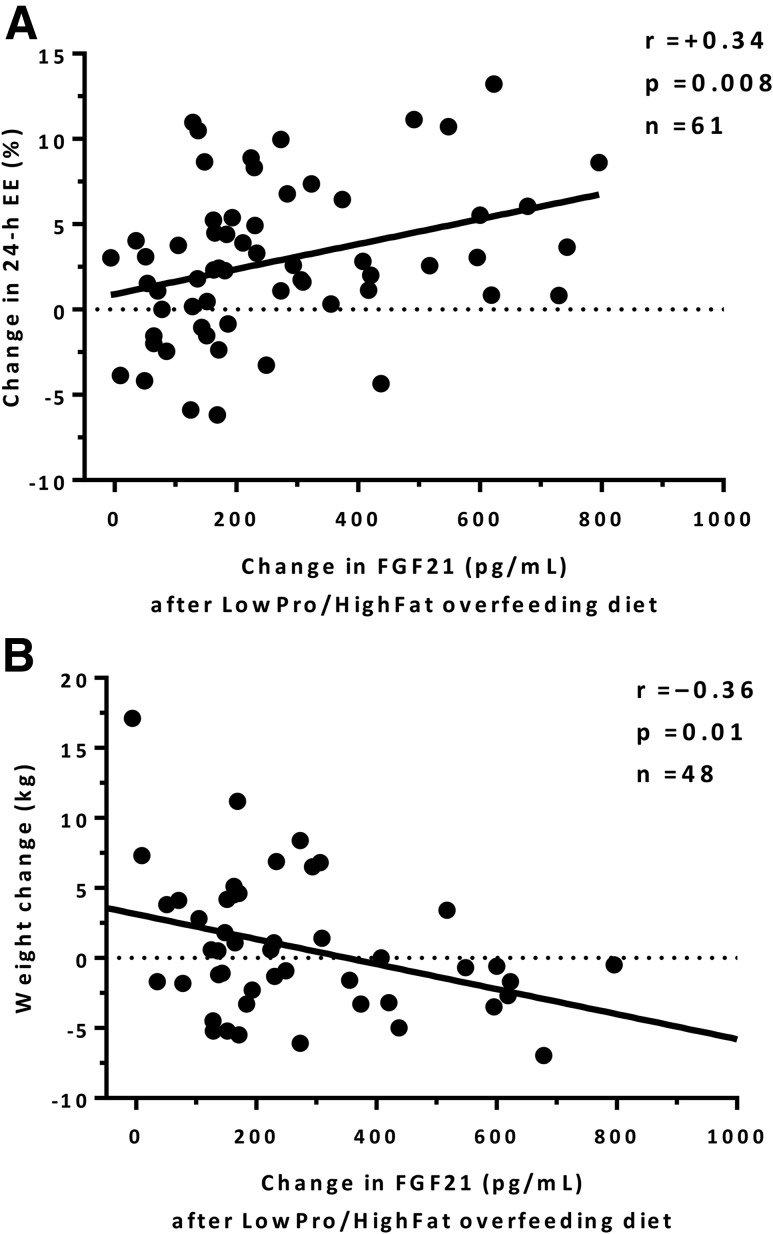
On average, FGF21 concentrations greatly increased after the two low-protein overfeeding diets, with a nearly threefold increase both after the LowPro/HighFat (+297%, CI 254–347) and LowPro/HighCHO (+326%, CI 234–456) overfeeding (Fig. 1A and B). The individual increases in FGF21 observed after these two low-protein overfeeding diets were correlated in the same subject (r = 0.78, P < 0.001) (Supplementary Fig. 5). Conversely, FGF21 concentrations decreased after 24-h fasting (−34%, CI −21 to −44) and all normal-protein overfeeding diets (Table 2), with the largest decrease after high-protein overfeeding (−75%, CI −66 to −81). A greater increase in FGF21 concentration after LowPro/HighFat overfeeding was associated with greater increase in 24-h EE (r = 0.34, P = 0.008) (Fig. 2A) (in men only: r = 0.31, P = 0.03), but not during any normal-protein overfeeding diet or fasting (P = 0.32) (Supplementary Fig. 6). There were no associations between the change in FGF21 after LowPro/HighFat overfeeding and 24-h RQ, macronutrient oxidation rates, or substrate balance (all P > 0.05).
Figure 1.
Plasma FGF21 concentrations before and after 24-h dietary interventions. A: Plasma FGF21 concentrations before and after each dietary intervention. Bars represent geographic means with 95% CIs. B: Individual changes in plasma FGF21 concentration after each dietary intervention. The red lines represent arithmetic means with 95% CIs. Macronutrient composition of diets: energy balance diet and NormalPro/NormalCHO overfeeding diet: 50% carbohydrate, 30% fat, 20% protein; NormalPro/HighCHO overfeeding diet: 75% carbohydrate, 5% fat, 20% protein; NormalPro/HighFat overfeeding diet: 20% carbohydrate, 60% fat, 20% protein; HighPro/HighFat overfeeding diet: 26% carbohydrate, 44% fat, 30% protein; LowPro/HighFat overfeeding diet: 51% carbohydrate, 46% fat, 3% protein; and LowPro/HighCHO overfeeding diet: 75% carbohydrate, 22% fat, 3% protein.
Figure 2.
Relationships between the change in plasma FGF21 concentration after 24-h low-protein high-fat overfeeding and the percentage change in 24-h EE from energy balance (A) and free-living weight change after 6 months (B). The macronutrient composition of LowPro/HighFat overfeeding diet was: 51% carbohydrate, 46% fat, and 3% protein. The percentage change in 24-h EE was calculated as: (24-h EEoverfeeding diet − 24-h EEenergy balance)/24-h EEenergy balance × 100. One subject found to have impaired fasting glucose on the OGTT and one subject with benign glycosuria were excluded from these analyses because these conditions are known to affect 24-h EE. Associations were quantified by the Pearson correlation index.
Despite a wide variability in weight change (SD 4.7 kg), on average, body weight was stable after 6 months (mean ± SD, 0.8 ± 4.7 kg; P = 0.26). A greater increase in plasma FGF21 concentration after LowPro/HighFat overfeeding at baseline was associated with weight loss at the follow-up visit (r = −0.36, P = 0.01, R2 = 12.9%) (Fig. 2B) (in men only: r = −0.41, P = 0.008), such that a 100 ng/mL increase in FGF21 after LowPro/HighFat overfeeding was associated with an average weight change of −0.9 kg (CI −1.5 to −0.2) at follow-up. Similarly, the change in 24-h EE during LowPro/HighFat overfeeding, but not during any other dietary intervention (all P > 0.05), was inversely associated with weight change (r = −0.30, P = 0.04). In multivariate analysis, however, only the change in FGF21 after LowPro/HighFat overfeeding (P = 0.04), but not the concomitant change in 24-h EE (P = 0.16), was the only predictor of weight change independently of age (P = 0.21), sex (P = 0.77), and ethnicity (P = 0.12).
Discussion
We aimed to test whether FGF21 mediates the change in 24-h EE observed during low-protein overfeeding, because we had previously shown that this diet identifies a metabolic phenotype resistant to weight gain. Circulating FGF21 concentrations increased acutely (i.e., after 24 h) and consistently after two different overfeeding diets with low-protein content (3%), while decreasing after fasting and other normal/high-protein overfeeding diets. Importantly, the increase in plasma FGF21 concentration after low-protein overfeeding was associated with diet-related changes in 24-h EE, where a greater increase in FGF21 concentration was associated with a higher increase in EE. We further determined that the extent of the increase in FGF21 concentration after LowPro/HighFat overfeeding was associated with weight change at 6 months, indicating that a decreased capability to increase FGF21 concentration in response to low-protein overfeeding is a hormonal feature of the thrifty metabolic phenotype inclined to gain weight over time.
The “thrifty” and “spendthrift” metabolic phenotypes hypothesis has evolved over the years from a theorized genotype that led to insulin overproduction due to food consumption favoring adipose storage (19), to a focus on the energy conservation in face of repeated famine or overeating (5,20,21). These human metabolic phenotypes can be described by the individual ability to increase or decrease EE in an energetically restricted (fasting) or unrestricted (overeating) setting. Although the extent of EE increase during overfeeding is highly dependent on the macronutrient composition of the diet (22), certain diets, such as low-protein overfeeding, appear more likely to uncover these metabolic phenotypes associated with weight change (5). In the current study including healthy subjects with normal glucose regulation, FGF21 concentrations increased in nearly all subjects by approximately threefold after 1 day of low-protein overfeeding, in line with what was reported in previous studies (10,13). Importantly, because the degree of this increase correlated with the dietary-related EE, we have identified one of the hormonal mediators of the EE response to this diet that characterizes the thrifty metabolic phenotype prone to weight gain. In addition, the ability to increase circulating FGF21 after low-protein overfeeding was associated with less weight gain or weight loss 6 months after subjects return to their routine activities and explained 12.9% of the interindividual variance in free-living weight change, a value much higher than that of established metabolic determinants of weight change such as lower 24-h EE (2.5%) and higher RQ (5.8%) during energy balance (23,24).
The biological mechanisms by which FGF21 may increase diet-induced EE during low-protein overfeeding in humans are not known but could involve UCP2 and UCP3, because FGF21 treatment of cultured human cardiomyocytes increases expression of UCP2 and UCP3 (25). Notably, the significant intrasubject variance (25%) (Supplementary Fig. 7) in FGF21 concentrations suggests that the capacity of increasing FGF21 in response to a low-protein diet is an individual-specific characteristic, perhaps genetically determined, which could partly explain the lower FGF21 concentrations found in blacks.
Our study has some limitations. First, we have a relatively small representation of women; therefore, our results may need to be validated in a larger female cohort. However, sensitivity analyses including only men provided similar results. Second, we do not have assessment of free-living food intake, physical activity, or fitness during the follow-up period; thus, we were not able to assess whether the association between FGF21 concentration and weight change was independent of these factors. Nevertheless, subjects were recruited as being weight-stable for 6 months before admission, and on average, weight did not change after 6 months, thus indicating that no substantial changes in free-living physical activity or food intake took place during the follow-up period.
In conclusion, we have identified a thrifty metabolic phenotype that can be characterized by reduced FGF21 response after 24 h of low-protein overfeeding and that confers susceptibility to weight gain. Furthermore, we have found that the increase in FGF21 after low-protein overfeeding is correlated with the diet-induced change in 24-h EE and, ultimately, with weight change. The present results are important in the context of our current obesogenic environment that includes the widespread overexposure to low-protein dietary options that are highly palatable, easily overeaten, and inexpensive, such as sodas, ice creams, doughnuts, etc. We speculate that exogenous FGF21 therapy may help metabolically thrifty individuals to prevent weight gain or achieving greater weight loss during obesity interventions. This may be useful for preventing and treating obesity in some people genetically prone to obesity and its complications.
Supplementary Material
Article Information
Acknowledgments. The authors thank the support staff of the Obesity and Diabetes Clinical Research Section and the participants of this study.
Funding. This research was supported by the Intramural Research Program of the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (DK-069029-11).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. K.L.V. contributed to data collection, analysis, and interpretation; the literature search; and drafting the manuscript. B.B. contributed to data collection, analysis, and interpretation. C.B. contributed to data interpretation. M.W. contributed to hormone measurement and data interpretation. J.K. contributed to study design and data interpretation. P.P. contributed to study design, data analysis and interpretation, and revision of the manuscript. All authors read and approved the final manuscript. P.P. is the guarantor of this work, and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented in abstract form at ENDO 2018—The 100th Annual Meeting of the Endocrine Society, Chicago, IL, 16–20 March 2018.
Footnotes
Clinical trial reg. no. NCT00523627, clinicaltrials.gov
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db18-0696/-/DC1.
See accompanying article, p. 266.
References
- 1.Sims EA, Danforth E Jr, Horton ES, Bray GA, Glennon J, Salans LB. Endocrine and metabolic effects of experimental obesity in man. Recent Prog Horm Res 1973;29:457–496 [DOI] [PubMed] [Google Scholar]
- 2.Piaggi P, Vinales KL, Basolo A, Santini F, Krakoff J. Energy expenditure in the etiology of human obesity: spendthrift and thrifty metabolic phenotypes and energy-sensing mechanisms. J Endocrinol Invest 2018;41:83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stock MJ. Gluttony and thermogenesis revisited. Int J Obes Relat Metab Disord 1999;23:1105–1117 [DOI] [PubMed] [Google Scholar]
- 4.Dulloo AG, Jacquet J. Low-protein overfeeding: a tool to unmask susceptibility to obesity in humans. Int J Obes Relat Metab Disord 1999;23:1118–1121 [DOI] [PubMed] [Google Scholar]
- 5.Schlögl M, Piaggi P, Pannacciuli N, Bonfiglio SM, Krakoff J, Thearle MS. Energy expenditure responses to fasting and overfeeding identify phenotypes associated with weight change. Diabetes 2015;64:3680–3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bray GA, Smith SR, de Jonge L, et al. . Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA 2012;307:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coskun T, Bina HA, Schneider MA, et al. . Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008;149:6018–6027 [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Lloyd DJ, Hale C, et al. . Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 2009;58:250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher FM, Maratos-Flier E. Understanding the physiology of FGF21. Annu Rev Physiol 2016;78:223–241 [DOI] [PubMed] [Google Scholar]
- 10.Laeger T, Henagan TM, Albarado DC, et al. . FGF21 is an endocrine signal of protein restriction. J Clin Invest 2014;124:3913–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill CM, Laeger T, Albarado DC, et al. . Low protein-induced increases in FGF21 drive UCP1-dependent metabolic but not thermoregulatory endpoints. Sci Rep 2017;7:8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laeger T, Albarado DC, Burke SJ, et al. . Metabolic responses to dietary protein restriction require an increase in FGF21 that is delayed by the absence of GCN2. Cell Reports 2016;16:707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maida A, Zota A, Sjøberg KA, et al. . A liver stress-endocrine nexus promotes metabolic integrity during dietary protein dilution. J Clin Invest 2016;126:3263–3278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bray GA, Redman LM, de Jonge L, et al. . Effect of protein overfeeding on energy expenditure measured in a metabolic chamber. Am J Clin Nutr 2015;101:496–505 [DOI] [PubMed] [Google Scholar]
- 15.Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr 2007;86:625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association Classification and diagnosis of diabetes. Sec 2. In Standards of Medical Care in Diabetes–2016 [published correction appears in Diabetes Care 2016;39:1653]. Diabetes Care 2016;39(Suppl. 1):S13–S22 [DOI] [PubMed] [Google Scholar]
- 17.Thearle MS, Pannacciulli N, Bonfiglio S, Pacak K, Krakoff J. Extent and determinants of thermogenic responses to 24 hours of fasting, energy balance, and five different overfeeding diets in humans. J Clin Endocrinol Metab 2013;98:2791–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest 1986;78:1568–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet 1962;14:353–362 [PMC free article] [PubMed] [Google Scholar]
- 20.Reinhardt M, Thearle MS, Ibrahim M, et al. . A human thrifty phenotype associated with less weight loss during caloric restriction. Diabetes 2015;64:2859–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weyer C, Vozarova B, Ravussin E, Tataranni PA. Changes in energy metabolism in response to 48 h of overfeeding and fasting in Caucasians and Pima Indians. Int J Obes Relat Metab Disord 2001;25:593–600 [DOI] [PubMed] [Google Scholar]
- 22.Vinales KL, Schlögl M, Piaggi P, et al. . The consistency in macronutrient oxidation and the role for epinephrine in the response to fasting and overfeeding. J Clin Endocrinol Metab 2017;102:279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zurlo F, Lillioja S, Esposito-Del Puente A, et al. . Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol 1990;259:E650–E657 [DOI] [PubMed] [Google Scholar]
- 24.Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. J Clin Endocrinol Metab 2013;98:E703–E707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Planavila A, Redondo-Angulo I, Ribas F, et al. . Fibroblast growth factor 21 protects the heart from oxidative stress. Cardiovasc Res 2015;106:19–31 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.