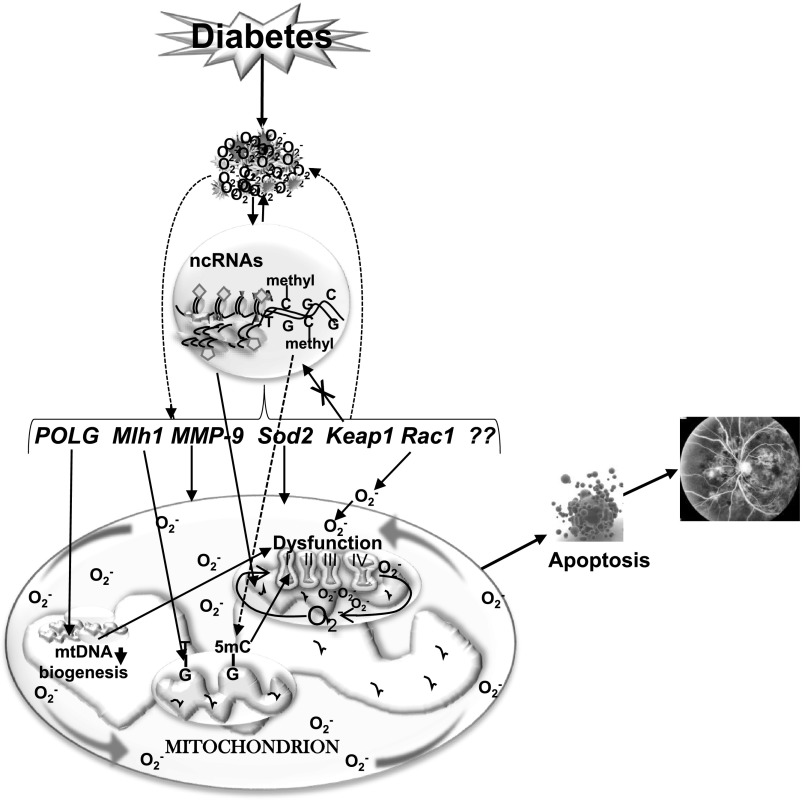
Figure 2.
Epigenetics and diabetic retinopathy. Increased ROS affect the epigenetic machinery, and the activities of enzymes responsible for maintaining DNA and histone methylation and histone acetylation are altered. Due to dynamic DNA methylation and histone modifications, gene expressions of MMP-9 and Rac1 are increased and that of Mlh1 and Sod2 are decreased. Furthermore, due to epigenetic modifications at the promoter of Keap1, a negative regulator of master transcription factor Nrf2, its expression is increased, and this impedes the translocation of Nrf2 inside the nucleus, compromising the antioxidant defense system. Hypermethylation of the POLG promoter further decreases its activity, and mtDNA replication is attenuated. mtDNA itself is also hypermethylated, and this decreases the transcription of mtDNA-encoded genes that are critical for functioning of the ETC complex. The compromised ETC system continues to fuel into a vicious cycle of free radicals, and release of cytochrome c in the cytosol activates apoptosis, ultimately resulting in the development of diabetic retinopathy.