Figure 4.
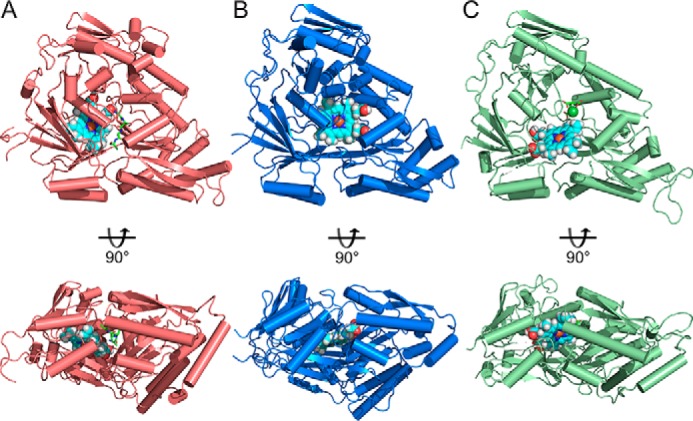
Comparing heme-docking studies of multiple Cluster C SBPs shows conserved heme-specific cleft in the substrate-binding pocket. Independent of canonical ligand size of these SBPs, structural conservation of Cluster C proteins creates flexible substrate-binding pockets large enough to accommodate heme binding. A, top-scoring model of heme docked in a heme-specific cleft of substrate-binding pocket of nthiOppA. The nthiOppA substrate-binding pocket is large enough to fit two substrates, heme and the co-purified peptide, shown in cyan and green, respectively. The docking model shows both ligands are fully enclosed and buried in the binding pocket. B, top-scoring model of gpHbpA (PDB code 3M8U) shows the docked heme in a relatively similar location in binding pocket. C, top-scoring heme docked model for ecNikA (PDB code 3DP8) also shows the substrate-binding pocket accommodates both heme and butane-1,2,4-tricarboxylate-chelated nickel, shown in green.
