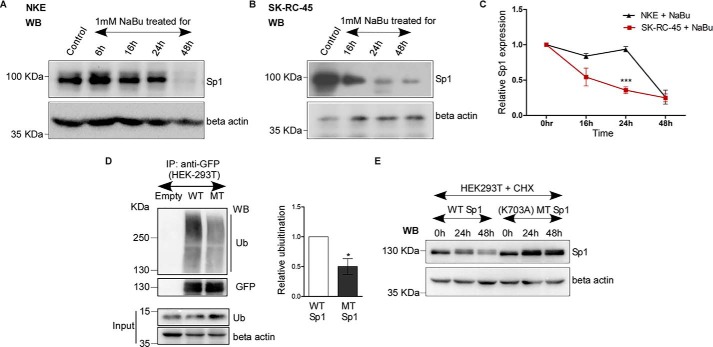
Figure 6.
Acetylated Sp1 degrades faster compared with less-acetylated Sp1 in response to butyrate treatment. A and B, NaBu treatment on NKE and SK-RC-45 cells down-regulates endogenous Sp1 protein expression. NKE and SK-RC-45 cells were treated with 1 mm NaBu up to 48 h, and cell extracts were prepared followed by Western blot analysis with antibodies against Sp1 and β-actin. WB, Western blotting. C, relative time-dependent loss of Sp1 protein expression by NaBu in SK-RC-45 is higher compared with NKE. Densitometric analysis of Western blotting was performed using ImageJ software. Error bars represent mean ± S.E. of three independent determinations (average ± S.E.; Student's t test; ***, p < 0.001). D, WT-Sp1 protein (WT) shows higher ubiquitination compared with mutated Sp1 (MT) protein (K703A). Cell extracts with IP buffer (see “Experimental Procedures”) from untransfected HEK-293T or co-transfected with indicated Sp1-GFP and ubiquitin expression plasmids in 3:1 ratio were immunoprecipitated (IP) with antibodies specific for GFP. Immunocomplexes and input were probed with ubiquitin, GFP and β-actin antibodies. Error bars represent mean ± S.E. of three independent determinations (average ± S.E.; Student's t test; *, p < 0.05) by densitometric analysis using ImageJ software. E, WT-Sp1 protein shows lower stability compared with Sp1 MT protein (K703A). HEK-293T were co-transfected with indicated Sp1-GFP and ubiquitin expression plasmids in 3:1 ratio. 24 h post transfection cells were treated with 100 μm cycloheximide (CHX) and chased up to next 48 h. Cell extracts were prepared with IP buffer (see “Experimental Procedures”) followed by Western blot analysis with antibodies against Sp1 and β-actin. The molecular mass of over-expressed Sp1 which is tagged with GFP is 125 KDa. WB, Western blotting.