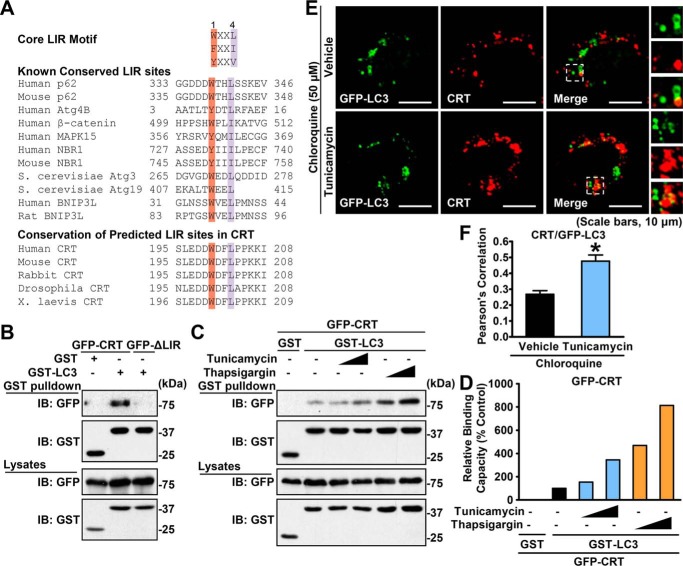
Figure 5.
Calreticulin physically interacts with the autophagic protein LC3 through the LIR motif. A, alignment of the LIR. B, calreticulin, but not its ΔLIR form, physically interacted with LC3. HEK293T cells were cotransfected with the indicated constructs. GSH-agarose beads were utilized to immunoprecipitate GST-tagged proteins, and immunoblotting (IB) was carried out to determine the coprecipitated proteins. C, the interaction between calreticulin and LC3 was increased under ER stress. HEK293T cells were cotransfected with the indicated constructs and treated with vehicle (DMSO) or gradient dosages of tunicamycin or thapsigargin for 16 h. GSH-agarose beads were then utilized to immunoprecipitate GST-tagged proteins, and immunoblotting was carried out to determine the coprecipitated proteins. D, densitometric quantification of coprecipitated GFP-calreticulin in C (normalized to GFP-calreticulin protein levels in cell lysates). Shown are representative images of immunofluorescence (scale bars = 10 μm). E and F, the colocalization of calreticulin and LC3 is enhanced in response to tunicamycin treatment. HeLa cells were transfected with myc-CRT and GFP-LC3 constructs and treated with tunicamycin (2 μg/ml) or vehicle (DMSO) for 6 h, followed by treatment with 50 μm chloroquine for an additional 10 h as indicated. myc-CRT was labeled with red fluorescence. Confocal microscopy analysis was performed. Representative cells (E) and quantification of Pearson's correlation coefficient for colocalization between CRT and GFP-LC3 (F) are shown. Data are presented as the mean ± S.E.; n = 5. *, p < 0.05 versus chloroquine and vehicle.