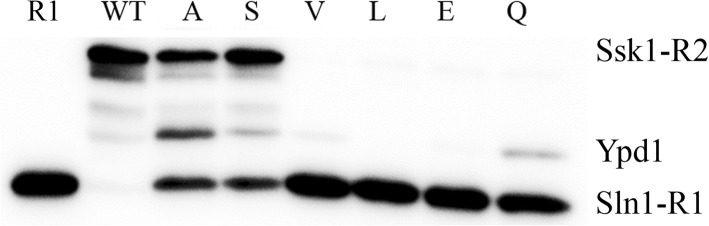
Fig. 3.
Residue in H + 4 position alters ability of Ypd1 to function as a phosphorelay intermediate. Phosphoryl transfer reactions contained equimolar concentrations of Sln1-R1, Ypd1, and Ssk1-R2 proteins. Reactions were quenched at 5 min with stop buffer containing EDTA and separated by SDS-PAGE. The gel bands were detected by phosphorimaging and analyzed using ImageJ software. The amount of radiolabel in Ssk1-R2 bands in the presence of Ypd1-G68X mutants was quantified and compared with the amount of Ssk1-R2 radiolabel in the presence of wild-type Ypd1 (normalized to 100%)