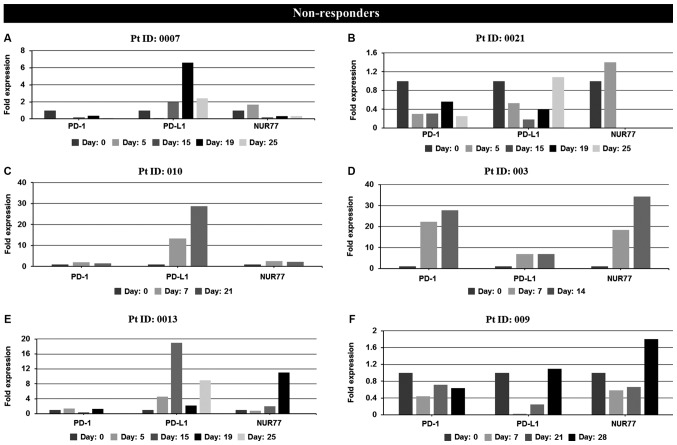
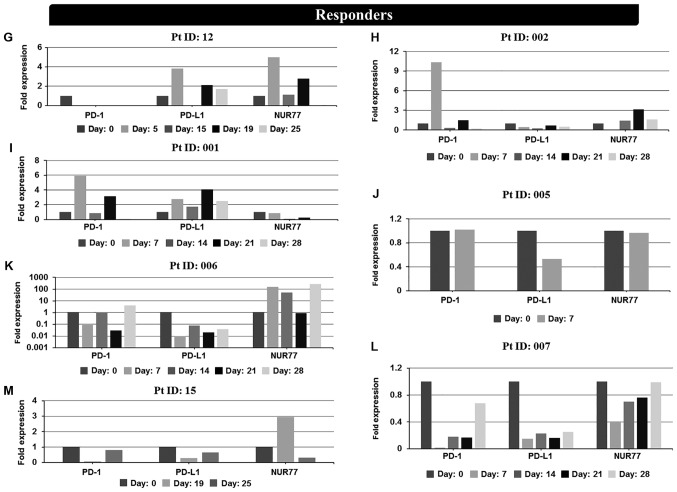
Figure 2.
In vivo mRNA expression profiles at screening and during cycle 1 of PD-1, PD-L1 and NUR77 from PBMC's of individual non-responder and responder patients treated with Aza alone or a combination of Aza and LBH589. Non-responders: (A) patient 0007 (resistant), (B) patient 0021 (resistant), (C) patient 010 (resistant), (D) patient 003 (resistant), (E) patient 0013 [stable disease (SD)], (F) patient 009 [progressive disease (PD)]. Day: 0=screening sample, Days 5–28=samples taken on specific day post screening. Responders: (G) patient 12 [partial response (PR)], (H) patient 002 (PR), (I) patient 001 [complete response (CR)], (J) patient 005 (CR), (K) patient 006 (CR), (L) patient 007 (CR), and (M) patient 015 [not evaluated (NE)]. Clinical response to treatment determined at 1, 3 and 6 months. Responses were defined according to international working group criteria for AML and MDS (15). Results for patient 006 expression levels are presented using a log scale. PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PBMC, peripheral blood mononuclear cells; Aza, azacytidine; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome.