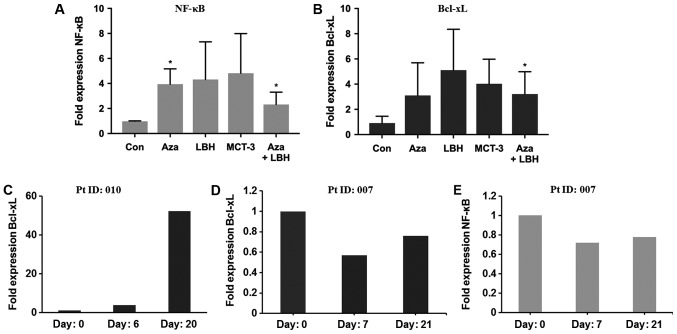
Figure 4.
In vitro and in vivo mRNA expression levels of NF-κB and Bcl-xL in KG-1 cells treated with Aza, LBH-589, MCT-3 or a combination of Aza+LBH-589 and from PBMC's from a non-responder and responder patient. (A) NF-κB and (B) Bcl-xL mRNA expression in KG-1 cells untreated (Con) or treated for 24 h with Aza, LBH-589 (LBH), MCT-3 or Aza+LBH *P<0.05 Con vs. Aza and Aza+LBH (n=3) and (C) Bcl-xL mRNA expression in PBMC's from non-responsive patient 010 at day 0, 6 and day 20 (n=1). (D) Bcl-xL and (E) NF-κB mRNA expression in PBMC's from an EGT-responsive patient 007 at day 0, 7 and day 21 (n=1). Responses were defined according to international working group criteria for AML and MDS (15). Aza, azacytidine; PBMC, peripheral blood mononuclear cells; EGT, epigenetic therapy; Con, control; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome.