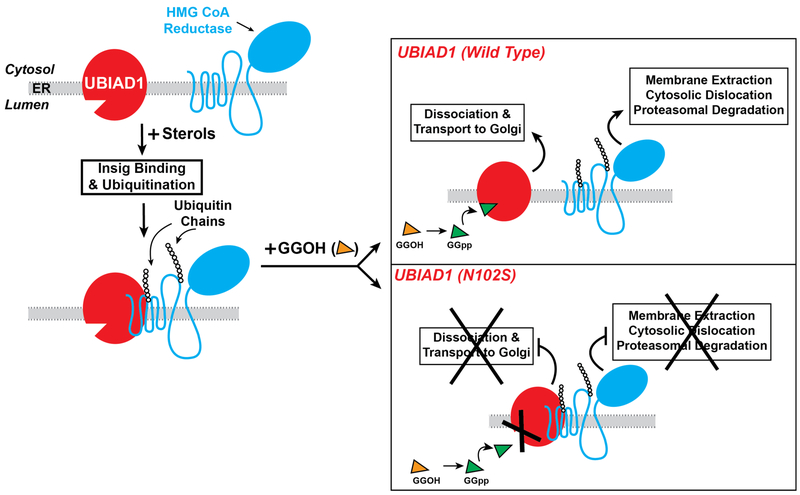
Figure 5.
Role of UBIAD1 in sterol-accelerated ERAD of HMG CoA reductase. The intracellular accumulation of sterols causes reductase to bind to Insigs, resulting in its ubiquitination by Insig-associated ubiquitin ligases and association with UBIAD1. Exogenously added GGOH becomes phosphorylated to produce GGpp, which enhances reductase ERAD by binding to UBIAD1 and stimulating release from reductase-Insig. This release allows for transport of UBIAD1 from ER to Golgi and membrane extraction, cytosolic dislocation, and proteasomal degradation of reductase. SCD-associated variants of UBIAD1 resist GGpp-induced release from reductase and remain in the ER to block reductase ERAD.