Abstract
Scope
Patients with persistent egg allergy have more immunoglobulin E (IgE) against sequential than conformational epitopes of ovomucoid (OVO). Here, we aimed to identify compounds capable to render sequential epitopes in egg.
Methods and results
Glutathione was used for in vitro reduction of OVO and circular dichroism analyses were performed. Glutathione reduced OVO in a concentration-dependent manner. Egg white was analyzed for reduced proteins with a thiol probe and by MALDI-TOF/TOF. In unprocessed total egg white, several reduced proteins were detected by the thiol probe, among them reduced ovalbumin could be confirmed with MS analyses.
Egg-allergics or sensitized controls were tested serologically (n = 19) for IgE against native and reduced OVO and in skin prick tests (n = 9). More patients had IgE against reduced than native OVO in Western blots. In skin prick test, five out of seven persistent egg-allergics and none of the controls reacted with reduced OVO.
Conclusion
Reduced egg proteins are present in natural egg white. Glutathione, which is present in egg and furthermore is used as texture-improving additive in processed food, is capable of reducing OVO. Patients with persistent egg allergy reacted rather to reduce the native OVO. Hence, our data indicate that reduction is a novel natural and processing-associated principle, which contributes to the allergenicity of food.
Keywords: Gal d 1, Glutathione, Ovomucoid, Persistent egg allergy, Reduction
1. Introduction
Allergy to egg has an estimated prevalence of 0.8–1.6% for children under 2.5 years [1–4], but is usually outgrown in later life [1, 5]. Sensitization to egg is often transient and low immunoglobulin E-levels (IgE-levels) to egg can occur without any symptoms [6]. The majority of allergic reactions are caused by egg white proteins [7] with ovomucoid (OVO, Gal d1) identified as most dominant major allergen [8, 9]. OVO is heavily glycosylated [10], consists of three Kazal-family inhibitory domains, is very stable due to its nine disulfide bridges [11], and remains monomeric even after extensive heating [12].
Cooke and Sampson reported different patterns of IgE reactivity to OVO with some patients preferentially recognizing conformational rather than linear epitopes [13]. Patients who do not outgrow egg allergy have IgE antibodies against four sequential epitopes on OVO, which are not recognized by patients with transient egg allergy [7].
The fact that linear epitopes are more important for adults might be explained by maturation of the barrier and enzymatic function of the gastrointestinal tract during infancy and adolescence, rendering effective peptide digestion [14]. On the other hand, complete cleavage may only occur upon extension of the whole protein. This requires reduction of the nine disulfide bonds of OVO in the first instance. There are, however, no known reducing conditions in the human intestine. This prompted us to search for natural or commercial available food additives, which could fulfill these significant molecular modifications.
We identified glutathione (GSH) as potential reducing agents. GSH is the most abundant nonprotein thiol compound in all living organisms being able to directly neutralize free radicals and reactive oxygen compounds [15, 16]. Since GSH serves as an immune booster, antioxidant, and detoxifier of xenobiotics, it has already been exploited in medicine, as a food additive, and in the cosmetic industry [17]. GSH is found in high levels not only in protein-rich foods such as milk [18] and eggs [19], but also in fresh legumes and fruits [20]. GSH addition in food and beverage products is self-limiting due to its sour taste and sulfur odor. The level of L-GSH in food ranges from 0.004 to 6.667%. In products containing eggs such as in baked goods and in pastries, GSH concentration varies from 0.250 to 0.333% [21].
Our data indicate that the antioxidant GSH is able to linearize OVO and as a consequence may foster sensitization. Indeed, in egg white reducing conditions in egg white are present suggesting that OVO might already exist in a reduced form in egg white. In addition, we investigated the qualitative differences in triggering IgE-crosslinking of conformationally intact versus reduced OVO exposing sequential IgE-epitopes in patients with persistent egg allergy.
2. Methods
2.1. Subjects
Serum of 20 subjects sensitized to egg was collected and stored at –20°C until use. All controls were tested for food allergens due to the underlying disease (either atopic dermatitis or unspecific food-related oral allergy symptoms in connection with allergic rhinitis). According to our standard procedure, those allergens to which sensitizations were found, e.g. egg, were then checked for their relevance in an open challenge condition by having the patients themselves making symptom-related diaries and monitoring their food intake. In all patients classified as controls, large quantities of eggs could be tolerated both in cooked as well as in partly heated form without any problems; thus, double-blind oral food challenges were not performed in these patients and they were declared as sensitized without any clinical symptoms. Subjects were considered egg-allergic based on the past medical history and oral provocation tests or the results of previous allergic reactions after consuming products, which, unbeknown to the patient, had contained egg. Blood of allergic and sensitized subjects was taken immediately after consultation with the hospital due to atopic dermatitis or food-related allergic symptoms in connection with allergic rhinitis.Patients were grouped into clinically reactive patients and “sensitized-only” patients as depicted in Table 1. Nine patients with egg-specific IgE were also tested in skin prick test (SPT) for OVO or its reduced and alkylated counterpart. Subjects were recruited with informed consent and institutional review board approval from the Clinic for Dermatology, Venereology and Allergology at the Charité Hospital in Berlin and from the Department of Dermatology at the Medical University of Vienna. The attributes of these human individuals are summarized in Table 1.
Table 1. Clinical characteristics of patients.
| Western Blot | SPT | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient Number | Sex | Age | Symptoms after consumption of egg products | OFC | sIgE egg white (kUA/L) | total IgE (kU/L) | OVO | ovora | OVO | ovora | Whole egg |
| Patients with hen egg allergy | |||||||||||
| 8 | w | 6 | Angioedema, urticaria | n.d.a) | 22 | >5000 | +++ | + | n.d. | n.d. | ++ |
| 5 | w | 19 | OAS | n.d.a) | 1.1 | 1023 | ++ | ++ | − | − | + |
| 20 | m | 21 | Anahylaxie (angioedema, abdominal pain, nausea) | angioedema. abdominal pain, vomiting, global sensation and dysphagie | 3.66 | 782 | ++ | + | n.d. | n.d. | +++ |
| 2 | w | 24 | Diarrhea, abdominal pain | n.d.a) | 7.29 | 2055 | +++ | +++ | + | ++ | +++ |
| 6 | m | 27 | Angioedema, urticaria | angioedema, urticaria | 28.4 | >5000 | ++ | ++ | + | ++ | n.d. |
| 7 | m | 29 | OAS | n.d.a) | 9.62 | >5000 | + | + | − | − | + |
| 26 | f | 31 | Angioedema, dyspnoe | n.d.a) | n.d. | >5000 | +++ | +++ | − | ++ | + |
| 28 | F | 32 | abdominal pain | n.d.a) | 53.9 | >5000 | +++ | +++ | − | ++ | n.d. |
| 9 | W | 37 | OAS, dyspnea | n.d.a) | 7.07 | 701 | +++ | ++ | ++ | +++ | +++ |
| 14 | W | 41 | Angioedema, dyspnoe | n.d.a) | 3.74 | 913 | +++ | ++ | n.d. | n.d. | +++ |
| Controls sensitized to hen eggb) | |||||||||||
| 24 | M | 5 | No (AD) | n.d. | 0.37 | 74,7 | − | − | n.d. | n.d. | n.d. |
| 25 | F | 11 | No | n.d. | 0.46 | 287 | − | − | n.d. | n.d. | − |
| 12 | W | 22 | No | n.d. | 5.23 | n.d. | +++ | − | n.d. | n.d. | − |
| 13 | W | 30 | No | n.d. | 1.5 | 1501 | ++ | ++ | n.d. | n.d. | − |
| 16 | M | 36 | No | n.d. | 1.34 | 719 | − | − | n.d. | n.d. | − |
| 10 | W | 42 | No | n.d. | n.d. | 480 | + | + | − | − | − |
| 15 | W | 42 | No | n.d. | 1.37 | 4363 | − | + | n.d. | n.d. | − |
| 3 | M | 50 | No | n.d. | 1.24 | 1972 | + | + | − | − | − |
| 1 | M | 53 | No (AD) | n.d. | 1.75 | >5000 | +++ | ++ | n.d. | n.d. | n.d. |
| 11 | M | 75 | No | n.d. | 0.86 | >5000 | − | +++ | n.d. | n.d. | n.d. |
Symptoms based on past medical history, through the results of previous allergic reactions after consuming products that, unbeknown to the patient, had contained egg.
Controls were chosen based on positive sensitization upon routine IgE-testing for the suspicion of food allergy that then could be ruled out as clinically relevant in the case of hen’s egg after open challenge
OAS, oral allergy syndrome; OFC, oral food challenge; AD atopic dermatitis; OVO, ovomucoid, ovora, OVO reduced and alkylated ovomucoid; n.d., not done.
Patients who were tested for OVO and ovora (reduced and alkylated ovomucoid) in SPT are marked in light gray.
2.2. Reduction of OVO by GSH
Reduction was performed by adding reduced GSH (Sigma-Aldrich, St. Louis, MO, USA; added concentration: 1, 0.5, 0.05, 0.01 and 0.002 mg/mL) to 2 mg/mL OVO (Worthington Biochemical Corporation, Lakewood, NJ, USA) at room temperature. Immediately after, samples were mixed with 4× non-reducing sample buffer (40% glycerol, 10% SDS, 0.33M Tris pH6.8, 0.05% bromophenol blue) and cooked for 5 min, before applying on a 12% SDS-PAGE. Subsequently, bands were visualized by Coomassie staining. For the gel of Fig. 1, OVO was dissolved in RPMI medium for better resolution, whereas for the other experiments OVO was dissolved in water. Densiometric analysis of the bands were performed using the software Image J1.45s of the National Institutes of Health (NIH).
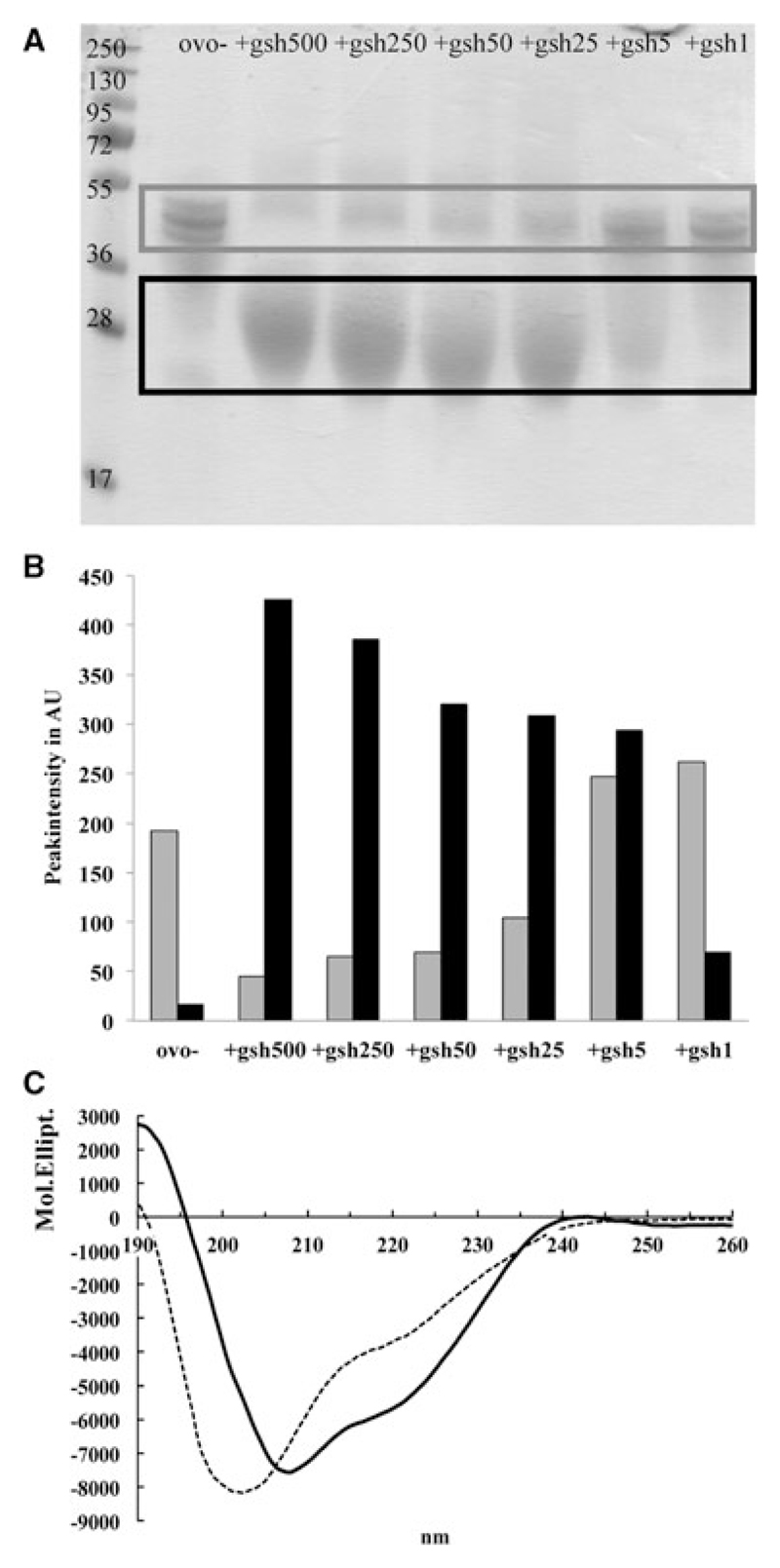
Figure 1.
Reduction of ovomucoid (OVO) with glutathione. (A) OVO was separated in the presence of glutathione in the w/w-ratio 2:1, 4:1, 10:1 by SDS-PAGE (under nonreducing condition) and stained with Coomassie. (B) Peak intensity of upper (gray box) and lower (black box) bands in the different lanes were analyzed using ImageJ software analysis. (C) Circular dichroism (CD) analysis of native (solid line) and glutathione-reduced and alkylated OVO (dashed line).
2.3. Reduction and alkylation of OVO
Stabilized reduced OVO was generated as previously described [22] by alkylation using as reduction agent either triethylphosphine or GSH (Sigma, Steinheim, Germany). The final concentration in the protein solution was 13 mM iodoethanol and 1.7 mM reducing agent, respectively. Samples were incubated for 60 min at 37°C under agitation and desalted by gel filtration. Protein content was determined at an OD of 280 nm.
2.4. Circular dichroism analysis of native as well as reduced and alkylated OVO
Circular dichroism (CD) measurements were performed on a Jasco J-715 spectropolarimeter with 100 μg/mL sample using a 1-mm path-length quartz cuvette equilibrated at 20°C as already described [23]. Spectra were recorded from 190 to 260 nm with 0.2-nm resolution at a scan speed of 50 nm/min and resulted from the average of five scans. The final spectra were corrected by subtracting the corresponding baseline spectrum obtained under identical conditions. Results were expressed as the mean residue ellipticity (Θ) at a given wavelength.
2.5. Detection of reduced egg proteins in raw egg white
Egg whites from fresh (stored <7 days) or old eggs (stored for 6–8 wk) were diluted 1:20 with distilled water to a concentration of approximately 5 mg/mL protein, homogenized for 1 min at 13 500 rpm and 2 μM fluorescent-labeled alkylation agent (5-IAF, Molecular Probes, Eugene, OR, USA), which reacts with free thiol groups, was added for 1 h at 37°C under agitation. As control, 5 mg/mL ovalbumin (OVA) and 5 mg/mL OVO were also treated with 2 μM 5-IAF in the presence or absence of the reducing agent. Unbound agent was removed by gel filtration. Quality of eluats was controlled by 12% SDS-PAGE under nonreducing conditions. Fluorescence bands were visualized using a UV-transilluminator (Herolab, Wiesloch, Germany). Gels were further stained by Coomassie.
2.6. Western blot analysis
Conformationally intact, reduced, or reduced and alkylated OVO were run on a preparative 12% SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes. After blocking with 5% dry milk in tris-buffered saline containing 0.05% Tween-20 (TBS-T), membranes were cut into strips, incubated overnight with 1:20 diluted sera from 19 patients, following incubation with horseradish peroxidase-conjugated goat anti-human IgE (KPL, Gaithersburg, MD, USA) and bands were visualized using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL, USA). Stripes were then exposed to an X-ray film (Thermo Scientific). Between each step, washing was performed with TBS-T.
Similarly, for the anti-OVO antibody Western blot, OVO, OVA, ovotransferrin, and natural egg white were applied in the presence or absence of GSH as a reducing agent (end concentration 0.2% w/v) on a 12% SDS-PAGE. After transfer onto a PVDF membrane and blocking with 5% dry milk in TBS-T, the membrane was incubated for 3 h with rabbit anti-OVO antibody (Abbomax, San Jose, CA, USA), followed by detection with horseradish-peroxidase-conjugated donkey-anti-rabbit-IgG (GE Healthcare, Vienna, Austria) diluted 1:2500, using the same chemiluminescent substrate as above. Membrane was then exposed to an X-ray film. Between each step, washing was performed with TBS-T.
2.7. Skin prick tests
Skin prick tests (SPTs) were performed with a sterile prick needles. Wheal size of egg and histamine response were measured after 20 min and expressed as a ratio to exclude variations due to different personnel or devices. In seven patients, alkylated serum albumin was included as additional negative control in SPT. SPTs were performed in two different centers by trained personnel. Color intensity of reactions to samples compared to the histamine-control was also measured [24].
3. Results
3.1. GSH reduces OVO in vitro
Since patients with persistent egg allergy preferentially have IgE against sequential epitopes, we sought to investigate ingredients in food with reducing potential. As depicted in Fig. 1A and B, GSH reduces OVO in a concentration-dependent manner, rendering a lower band at approximately 30 kDa (black box in Fig. 1A and black bars in Fig. 1B), whereas unreduced OVO showed a band at approximately 45 kDa (gray box in Fig. 1A and gray bars in Fig. 1B). Densiometric analyses clearly showed that increasing amounts of GSH increase the intensity of the reduced OVO (lower bands, black bars), whereas the relative amount of native OVO, upper band (gray bars), diminishes.
Since reduced proteins might refold in absence of reducing agents, reduced OVO was stabilized by alkylation [7] for further structural analysis. CD analysis revealed a shift of the two minima of native OVO toward a single minimum measured at 200 nm (Fig. 1B) indicating loss of the secondary structure. Hence, our data demonstrate that GSH is able to reduce OVO.
3.2. Reduced egg proteins are found in natural egg white
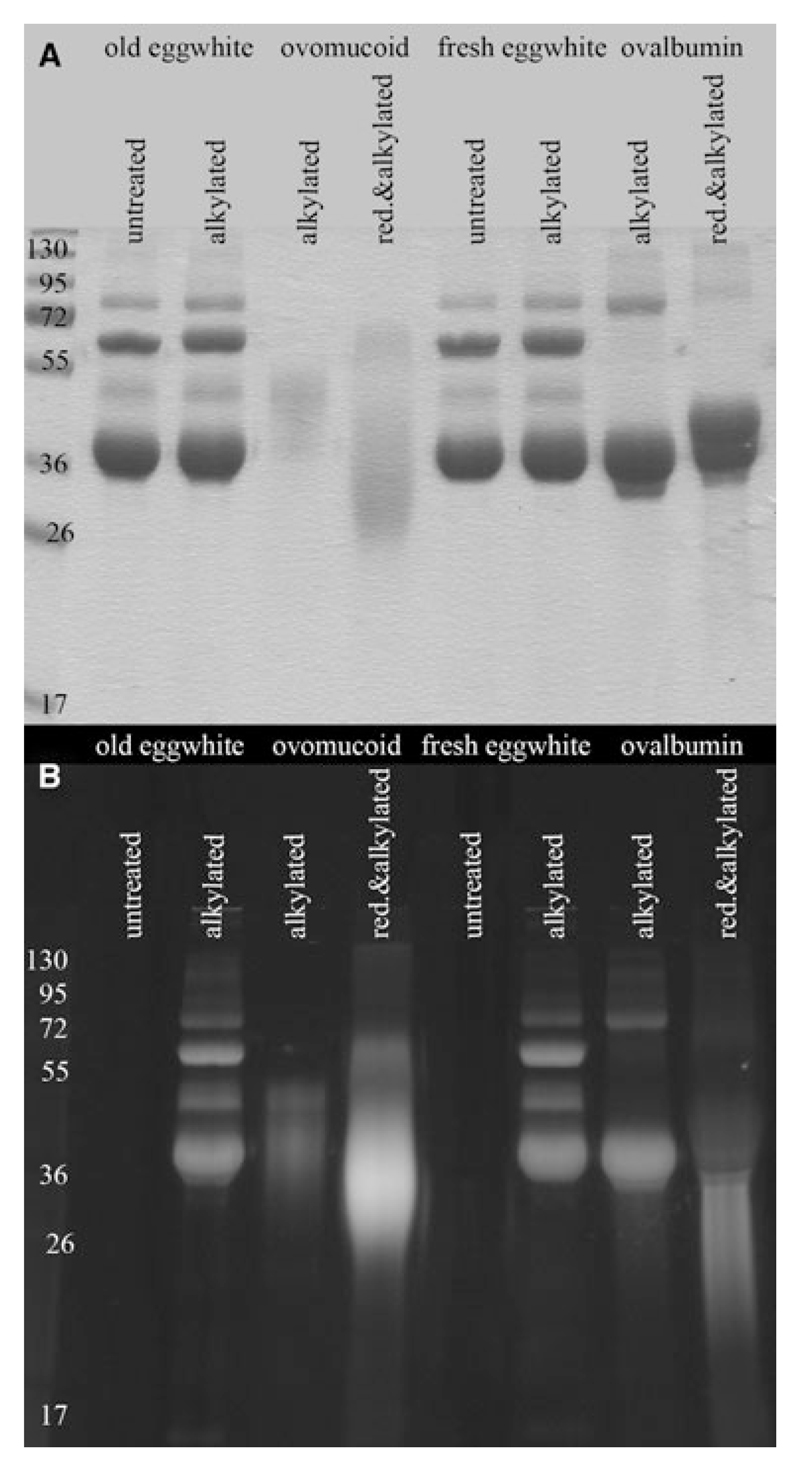
To test whether OVO occurs in a reduced state in egg itself, we sought to detect linearized egg proteins in old and fresh eggs. Hence, a fluorescent probe that only recognized free thiol groups was added to egg white and applied on a gel. As depicted in Fig. 2B, all bands were labeled by this probe indicating the presence of reduced proteins in egg white.
Figure 2.
Analysis of reduced ovomucoid (OVO) in fresh and old egg white. (A) Egg white derived from new and old eggs was separated by SDS-PAGE under nonreducing conditions and stained by Coomassie and (B) free thiol groups of reduced OVO were detected by addition of fluorescence-labeled probe to egg white. Proteins labeled with the probe were visualized using a UV-transilluminator.
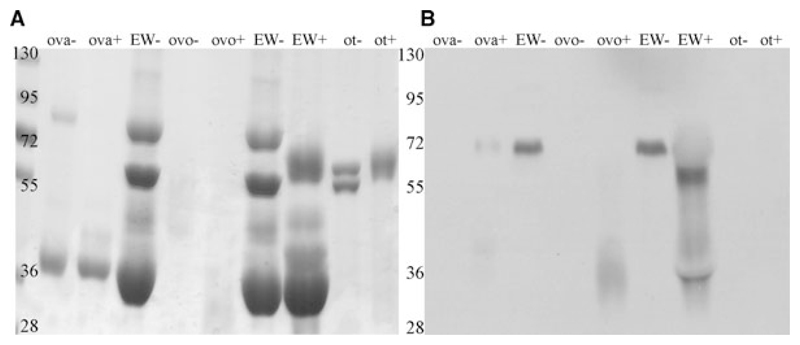
OVA and OVO ran similarly in SDS-PAGE around 30 kDa and in total egg white a single band at a height of 70 kDa was recognized by a putative OVO-specific antibody (Fig. 3A and B). Therefore, we were not able to differentiate between the two, even not when applying MS analyses that only identified OVA (Supporting Information). This might be due to the heavy glycosylation of OVO [10, 11], or to the dominance of OVA. Glycosylation is a well-known methodological problem in MS [25]. Further, no differences between old and fresh eggs were detected by SDS-PAGE.
Figure 3.
Coomassie and Western blot with anti-OVO antibody. (A) Ovalbumin (OVA), ovomucoid (OVO), ovotransferrin (ot), and egg white (EW) were analyzed by SDS-PAGE in the absence (–) or presence (+) of glutathione. (A) Protein-staining by Coomassie. (B) Western blot with epitope-purified rabbit anti-OVO antibody.
Nevertheless, the fact that the OVA is present in a reduced form in egg white suggested that other egg proteins might be similarly present in a reduced form. We hypothesized that food processing factors that cause reduction of OVO might promote exposure of sequential epitopes.
3.3. Patients with persistent egg allergy recognize reduced rather than native OVO
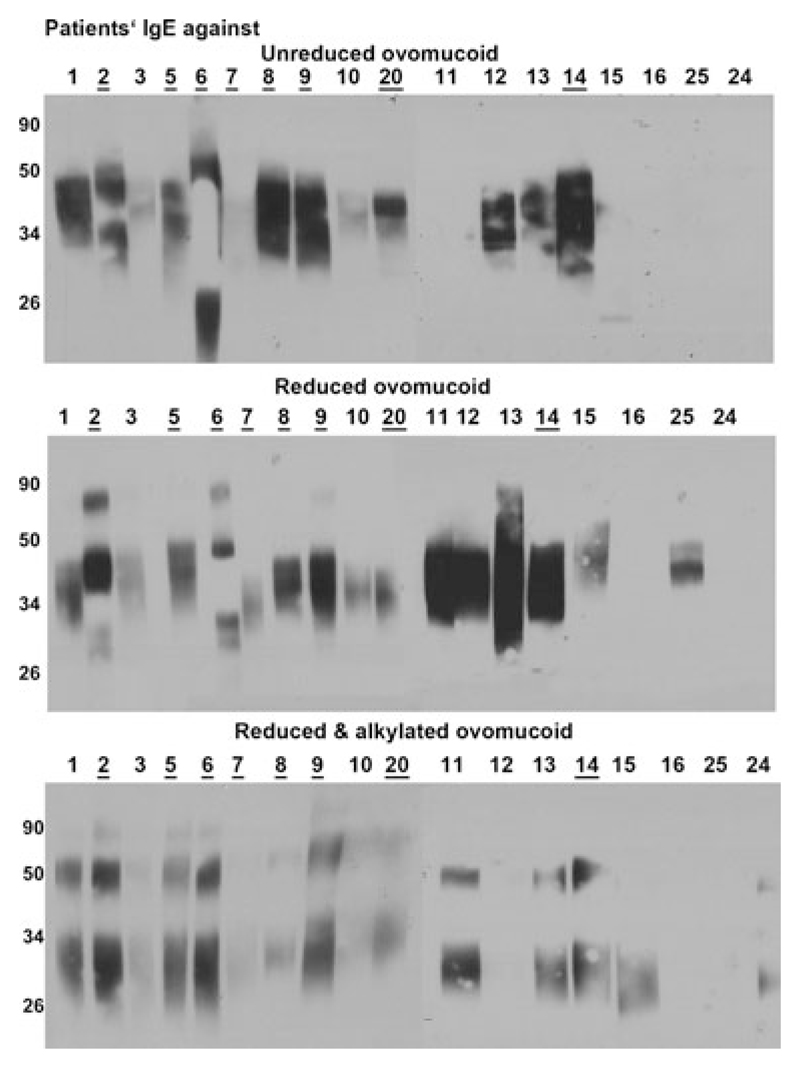
In order to evaluate the potential impact of reduction on IgE binding to OVO, we first subjected native and reduced OVO to Western blot analysis. Typically, IgE binding revealed rather fuzzy bands due to heavy glycosylation of OVO [10, 11]. IgE binding to native OVO was observed for 15 of 19 egg-allergic patients, whereas 18 of 19 recognized reduced OVO (#11, 15, 25 in addition). Alkylation unfortunately negatively affected the IgE-epitopes, since alkylation abrogated IgE binding of patient #12 and #25 (Fig. 4A). Two patients (#11, #15) solely reacted to reduced, whereas patient #12 solely recognized native OVO. Therefore, at this point it seemed that for the majority of our patient cohort reduction of OVO was important for IgE binding.
Figure 4.
Ovomucoid specific IgE of patients’ serum in Western blot. Sera from 18 patients sensitized to eggs or with clinical reactivity (underlined) were tested for IgE against native, reduced, and reduced and alkylated OVO.
3.4. Qualitative assessment of SPTs to conformationally intact and stably reduced OVO
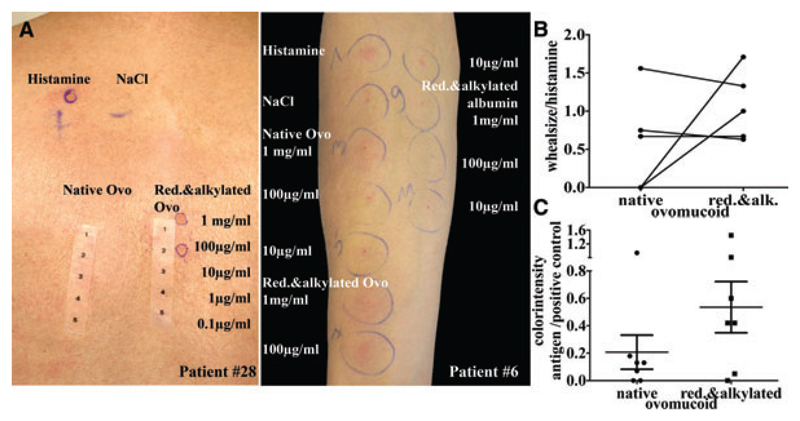
To compare the clinical relevance of reduced to native OVO; SPTs were performed with reduced and alkylated OVO to exclude the possibility of refolding. Nine individuals were recruited, from which seven still had clinical reactivity to egg and two not (Table 1). Patients with no clinical symptoms (#3, 10), as well as patients with oral allergy syndrome (#5, 7) had a negative prick to either form of OVO. Of the remaining five patients with clinical symptoms, three (#2, 6, 9) reacted to both, native, and linearized/reduced OVO and two (#26, 28) only to linearized allergen (Fig. 5A for representative examples). Patients’ wheals to reduced OVO appeared faster and were more intense in color, but wheal diameters were comparable to those achieved with native OVO (Fig. 5B). To exclude false positive reactions of IgE against alkylated protein, alkylated HSA was pricked in seven patients, which all remained negative. Hence, the results of the SPTs emphasize the importance of reduced OVO as a mediator in type I hypersensitivity reaction in patients with persistent egg-allergy.
Figure 5.
Skin prick reactions to native and reduced ovomucoid. (A) Skin prick tests (SPTs) of patients with persistent egg allergy were performed using samples as indicated on photos. Results of two patients with positive SPTs are shown as examples. SPT results were analyzed according to wheal size (B) and color intensity as objective parameters (C).
4. Discussion
Egg allergy is usually outgrown within the first years of life. However, a small percentage of individuals remain allergic. Earlier studies demonstrated that sera obtained from patients with persistent egg allergy had high IgE binding activity to pepsin-treated OVO [12] and that they usually had IgEs against sequential epitopes of OVO and OVA, which were not recognized by transient egg-allergic patients [7]. We defined two groups of patients either with persistent egg allergy or sensitized-only to egg. The median age among the egg-allergics was 28 years, thus much higher than the usual egg-allergic patient, which are children under the age of 5. The recruitment scheme was based on (i) patients’ history, (ii) length of interval between clinical reactivity and serum sampling, (iii) by high specific IgE-levels, and (iv) (not in all cases) by positive SPT and/or provocation (one case). The rational for relying mostly on (i)–(iii) derived from literature suggesting that high IgE-levels are predictors of clinical reactivity and can eliminate the need to perform oral challenge tests [26–30].
In contrast to inhaled allergens, the structure of food allergens can be affected by food processing, enzymatic digestion, and cooking prior to uptake [31–35]. Hence, we sought to explore circumstances, which could generate sequential epitopes. Given that the OVO fold is stabilized by nine disulfide bridges, we looked for antioxidants used in the food industry that favor reduction of OVO. We identified GSH with a potential role in this process. GSH is the most prominent antioxidant of every cell and as such also present in the hen oocyte. It is predominantly present in the reduced form and is a marker for oxidative stress in humans [36] with cellular concentrations ranging from 0.5 to 10 mM [37]. We provide evidence that GSH is capable of reducing OVO in a concentration-dependent manner in vitro and that egg-white proteins such as OVA are already present in a reduced form in egg white. The data with the fluorescent probe binding to free sulfhydryl-groups also suggests that other egg proteins such as OVO might be present in a reduced state. Since GSH is abundantly present in eggs [15, 19, 38–40], it will likely contribute to the reducing conditions in egg white. The observed difference found in the conformation between purified versus OVO in its natural context, has also been reported with other proteins, e.g. human α-lactalbumin, which changes its conformation upon purification [41, 42].
To our knowledge, this is the first study offering a mechanistic explanation how sensitization to sequential epitopes might occur. Methods in food preparation and food processing as well as food additives providing reducing conditions or stabilizing unfolded proteins may consequently promote sensitization to sequential epitopes of unfolded OVO.
In a next step, we investigated differences in skin prick reactions to reduced versus native OVO. In our study, 50% of our patients had egg white- and OVO-specific IgE without being clinical reactive to egg, underlining the fact that allergen-specific IgE is a marker of allergic sensitization and not of allergic disease. Nine individuals were recruited with IgE antibodies against egg white, two were considered sensitized-only, because of the lack of clinical symptoms. Of the seven egg-allergics, two had mild allergic symptoms (oral allergy syndrome) and had a negative prick to either native or reduced OVO (2 of 7). All remaining patients with clinical reactivity (5 of 7) had a positive prick against reduced OVO, but not always to native OVO (3 of 7). Hence, two egg-allergic patients solely reacted to unfolded OVO highlighting the fact that IgE against sequential epitopes were responsible for the allergic manifestation. Notably, positive prick reactions to native and reduced OVO seemed to be qualitatively different. Application of reduced OVO was associated with a faster and more intense wheal color compared to native OVO, even though wheal sizes between both types of OVO were comparable. The faster and more intense wheal color in the skin prick response to reduced OVO might be due to exposure of additional sequential IgE-epitopes or facilitated cross-linking potential compared to native OVO. Thus, we propose reduced OVO as an important sensitizer and elicitor of type I hypersensitivity reactions in patients with persistent egg allergy.
In conclusion, our data confirm that sequential rather than conformational epitopes are important in the induction of an allergic reaction in patients with persistent egg allergy. Importantly, we found evidence of reduced proteins in egg white and that GSH, as a likely natural source in egg, might be responsible for reduction of these proteins. In this respect, we showed a concentration-dependent reduction of OVO with GSH in vitro.
GSH is used in the food industry, since it softens the dough as reducing agent. It is especially applied for cakes and pastries, which often also contain eggs, to stop pastry textures becoming too heavy [43]. Hence, specifically the addition of GSH in food containing eggs might promote sensitization to sequential epitopes of OVO.
Supplementary Material
Additional supporting information may be found in the online version of this article at the publisher’s web-site
Acknowledgments
We thank Josefine Grünhagen from Charité – Universitätsmedizin Berlin for excellent clinical assistance and management and Mr. Michael Gartner and Ms. Durga Krishnamurthy, M. Sc., for helpful assistance in experiments. The work was supported by L467-B05, SFB F1808-B13, SFB 4606-B19 and CCHD APW01205FW of the FWF, Austrian Science Fund, and by NanoHealth 819721 of the FFG, Austrian Research Agency.
Abbreviations
- CD
circular dichroism
- GSH
glutathione
- OVA
ovalbumin
- OVO
ovomucoid
- SPT
skin prick test
- TBS
tris-buffered saline
Footnotes
The authors have declared no conflict of interest.
References
- [1].Dieguez MC, Cerecedo I, Muriel A, Zamora J, et al. Utility of diagnostic tests in the follow-up of egg-allergic children. Clin Exp Allergy: J Brit Soc Allergy Clin Immunol. 2009;39:1575–1584. doi: 10.1111/j.1365-2222.2009.03299.x. [DOI] [PubMed] [Google Scholar]
- [2].Eggesbo M, Botten G, Halvorsen R, Magnus P. The prevalence of allergy to egg: a population-based study in young children. Allergy. 2001;56:403–411. doi: 10.1034/j.1398-9995.2001.056005403.x. [DOI] [PubMed] [Google Scholar]
- [3].Osterballe M, Hansen TK, Mortz CG, Host A, et al. The prevalence of food hypersensitivity in an unselected population of children and adults. Pediatr Allergy Immunol. 2005;16:567–573. doi: 10.1111/j.1399-3038.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- [4].Venter C, Pereira B, Voigt K, Grundy J, et al. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy. 2008;63:354–359. doi: 10.1111/j.1398-9995.2007.01570.x. [DOI] [PubMed] [Google Scholar]
- [5].Hansen TK, Host A, Bindslev-Jensen C. An evaluation of the diagnostic value of different skin tests with egg in clinically egg-allergic children having atopic dermatitis. Pediatr Allergy Immunol. 2004;15:428–434. doi: 10.1111/j.1399-3038.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- [6].Benhamou AH, Caubet JC, Eigenmann PA, Nowak-Wegrzyn A, et al. State of the art and new horizons in the diagnosis and management of egg allergy. Allergy. 2010;65:283–289. doi: 10.1111/j.1398-9995.2009.02251.x. [DOI] [PubMed] [Google Scholar]
- [7].Jarvinen KM, Beyer K, Vila L, Bardina L, et al. Specificity of IgE antibodies to sequential epitopes of hen’s egg ovomucoid as a marker for persistence of egg allergy. Allergy. 2007;62:758–765. doi: 10.1111/j.1398-9995.2007.01332.x. [DOI] [PubMed] [Google Scholar]
- [8].Hoffman DR. Immunochemical identification of the allergens in egg white. J Allergy Clin Immunol. 1983;71:481–486. doi: 10.1016/0091-6749(83)90465-7. [DOI] [PubMed] [Google Scholar]
- [9].Langeland T. A clinical and immunological study of allergy to hen’s egg white. II. Antigens in hen’s egg white studied by crossed immunoelectrophoresis (CIE) Allergy. 1982;37:323–333. doi: 10.1111/j.1398-9995.1982.tb01918.x. [DOI] [PubMed] [Google Scholar]
- [10].Offengenden M, Fentabil MA, Wu J. N-glycosylation of ovomucin from hen egg white. Glycoconj J. 2011;28:113–123. doi: 10.1007/s10719-011-9328-3. [DOI] [PubMed] [Google Scholar]
- [11].Kato I, Schrode J, Kohr WJ, Laskowski M., Jr Chicken ovomucoid: determination of its amino acid sequence, determination of the trypsin reactive site, and preparation of all three of its domains. Biochemistry. 1987;26:193–201. doi: 10.1021/bi00375a027. [DOI] [PubMed] [Google Scholar]
- [12].Urisu A, Ando H, Morita Y, Wada E, et al. Allergenic activity of heated and ovomucoid-depleted egg white. J Allergy Clin Immunol. 1997;100:171–176. doi: 10.1016/s0091-6749(97)70220-3. [DOI] [PubMed] [Google Scholar]
- [13].Cooke SK, Sampson HA. Allergenic properties of ovomucoid in man. J Immunol. 1997;159:2026–2032. [PubMed] [Google Scholar]
- [14].Martos G, Lopez-Exposito I, Bencharitiwong R, Berin MC, et al. Mechanisms underlying differential food allergy response to heated egg. J Allergy Clin Immunol. 2011;127:990–997 e1–2. doi: 10.1016/j.jaci.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Des Roches A, Nguyen M, Paradis L, Primeau MN, et al. Tolerance to cooked egg in an egg allergic population. Allergy. 2006;61:900–901. doi: 10.1111/j.1398-9995.2006.01134.x. [DOI] [PubMed] [Google Scholar]
- [16].Willems AM, Nieuwboer A, Chavret F, Desloovere K, et al. The use of rhythmic auditory cues to influence gait in patients with Parkinson’s disease, the differential effect for freezers and non-freezers, an explorative study. Disabil Rehabil. 2006;28:721–728. doi: 10.1080/09638280500386569. [DOI] [PubMed] [Google Scholar]
- [17].Nici L, Donner C, Wouters E, Zuwallack R, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Resp Crit care Med. 2006;173:1390–1413. doi: 10.1164/rccm.200508-1211ST. [DOI] [PubMed] [Google Scholar]
- [18].Xu S, Yashchuk VV, Donaldson MH, Rochester SM, et al. Magnetic resonance imaging with an optical atomic magnetometer. Proc Natl Acad Sci USA. 2006;103:12668–12671. doi: 10.1073/pnas.0605396103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pan C, Zhao Y, Liao SF, Chen F, et al. Effect of seleniumenriched probiotics on laying performance, egg quality, egg selenium content, and egg glutathione peroxidase activity. J Agric Food Chem. 2011;59:11424–11431. doi: 10.1021/jf202014k. [DOI] [PubMed] [Google Scholar]
- [20].Evans RC, Douglas P, Williams JA, Rochester DL. A novel luminescence-based colorimetric oxygen sensor with a “traffic light” response. J Fluoresc. 2006;16:201–206. doi: 10.1007/s10895-005-0037-9. [DOI] [PubMed] [Google Scholar]
- [21].Kohjin C. GRAS notice for glutathione. 2009 http://www.access-data.fda.gov/scripts/fcn/gras_notices/grn0293.pdf.
- [22].Hale JE, Butler JP, Gelfanova V, You JS, et al. A simplified procedure for the reduction and alkylation of cysteine residues in proteins prior to proteolytic digestion and mass spectral analysis. Anal Biochem. 2004;333:174–181. doi: 10.1016/j.ab.2004.04.013. [DOI] [PubMed] [Google Scholar]
- [23].Resch Y, Weghofer M, Seiberler S, Horak F, et al. Molecular characterization of Der p 10: a diagnostic marker for broad sensitization in house dust mite allergy. Clin Exp Allergy. 2011;41:1468–1477. doi: 10.1111/j.1365-2222.2011.03798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Knight AK, Shreffler WG, Sampson HA, Sicherer SH, et al. Skin prick test to egg white provides additional diagnostic utility to serum egg white-specific IgE antibody concentration in children. J Allergy Clin Immunol. 2006;117:842–847. doi: 10.1016/j.jaci.2005.12.1304. [DOI] [PubMed] [Google Scholar]
- [25].Harvey DJ. Proteomic analysis of glycosylation: structural determination of N-and O-linked glycans by mass spectrometry. Expert Rev Proteomics. 2005;2:87–101. doi: 10.1586/14789450.2.1.87. [DOI] [PubMed] [Google Scholar]
- [26].Celik-Bilgili S, Mehl A, Verstege A, Staden U, et al. The predictive value of specific immunoglobulin E levels in serum for the outcome of oral food challenges. Clin Exp Allergy. 2005;35:268–273. doi: 10.1111/j.1365-2222.2005.02150.x. [DOI] [PubMed] [Google Scholar]
- [27].Montesinos E, Martorell A, Felix R, Cerda JC. Egg white specific IgE levels in serum as clinical reactivity predictors in the course of egg allergy follow-up. Pediatr Allergy Immunol. 2010;21:634–639. doi: 10.1111/j.1399-3038.2009.00940.x. [DOI] [PubMed] [Google Scholar]
- [28].Osterballe M, Bindslev-Jensen C. Threshold levels in food challenge and specific IgE in patients with egg allergy: is there a relationship? J Allergy Clin Immunol. 2003;112:196–201. doi: 10.1067/mai.2003.1603. [DOI] [PubMed] [Google Scholar]
- [29].Tripodi S, Businco AD, Alessandri C, Panetta V, et al. Predicting the outcome of oral food challenges with hen’s egg through skin test end-point titration. Clin Exp Allergy. 2009;39:1225–1233. doi: 10.1111/j.1365-2222.2009.03250.x. [DOI] [PubMed] [Google Scholar]
- [30].Hill DJ, Heine RG, Hosking CS. The diagnostic value of skin prick testing in children with food allergy. Pediatr Allergy Immunol. 2004;15:435–441. doi: 10.1111/j.1399-3038.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- [31].Breiteneder H, Clare Mills EN. Plant food allergens–structural and functional aspects of allergenicity. Biotechnol Adv. 2005;23:395–399. doi: 10.1016/j.biotechadv.2005.05.004. [DOI] [PubMed] [Google Scholar]
- [32].Furmonaviciene R, Shakib F. The molecular basis of allergenicity: comparative analysis of the three-dimensional structures of diverse allergens reveals a common structural motif. Mol Pathol. 2001;54:155–159. doi: 10.1136/mp.54.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lehrer SB, Ayuso R, Reese G. Current understanding of food allergens. Ann NY Acad Sci. 2002;964:69–85. doi: 10.1111/j.1749-6632.2002.tb04133.x. [DOI] [PubMed] [Google Scholar]
- [34].Teuber SS. Hypothesis: the protein body effect and other aspects of food matrix effects. Ann NY Acad Sci. 2002;964:111–116. doi: 10.1111/j.1749-6632.2002.tb04136.x. [DOI] [PubMed] [Google Scholar]
- [35].Roth-Walter F, Berin MC, Arnaboldi P, Escalante CR, et al. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer’s patches. Allergy. 2008;63:882–890. doi: 10.1111/j.1398-9995.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- [36].Knapen MF, Zusterzeel PL, Peters WH, Steegers EA. Glutathione and glutathione-related enzymes in reproduction. A review. Eur J Obstet Gynecol Reprod Biol. 1999;82:171–184. doi: 10.1016/s0301-2115(98)00242-5. [DOI] [PubMed] [Google Scholar]
- [37].Meister A. Glutathione metabolism and its selective modfication. J Biol Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- [38].Ebeid TA. The impact of incorporation of n-3 fatty acids into eggs on ovarian follicular development, immune response, antioxidative status and tibial bone characteristics in aged laying hens. Animal. 2011;5:1554–1562. doi: 10.1017/S1751731111000619. [DOI] [PubMed] [Google Scholar]
- [39].Fahey RC, Mikolajczyk SD, Meier GP, Epel D, Carroll EJ., Jr The glutathione thiol-disulfide status in the sea urchin egg during fertilization and the first cell division cycle. Biochim Biophys Acta. 1976;437:445–453. doi: 10.1016/0304-4165(76)90013-1. [DOI] [PubMed] [Google Scholar]
- [40].Emly M, Rochester P. A new look at constipation management in the community. Brit J Community Nurs. 2006;11:326. doi: 10.12968/bjcn.2006.11.8.21664. 328–332. [DOI] [PubMed] [Google Scholar]
- [41].Hakansson AP, Roche-Hakansson H, Mossberg AK, Svanborg C. Apoptosis-like death in bacteria induced by HAMLET, a human milk lipid-protein complex. PloS One. 2011;6:e17717. doi: 10.1371/journal.pone.0017717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mercer N, Ramakrishnan B, Boeggeman E, Qasba PK. Applications of site-specific labeling to study HAMLET, a tumoricidal complex of alpha-lactalbumin and oleic acid. PloS One. 2011;6:e26093. doi: 10.1371/journal.pone.0026093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Figoni PI. How Baking Works: Exploring the Fundamentals of Baking Science. John Wiley & Sons; Hoboken, New Jersey: 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.