Figure 1.
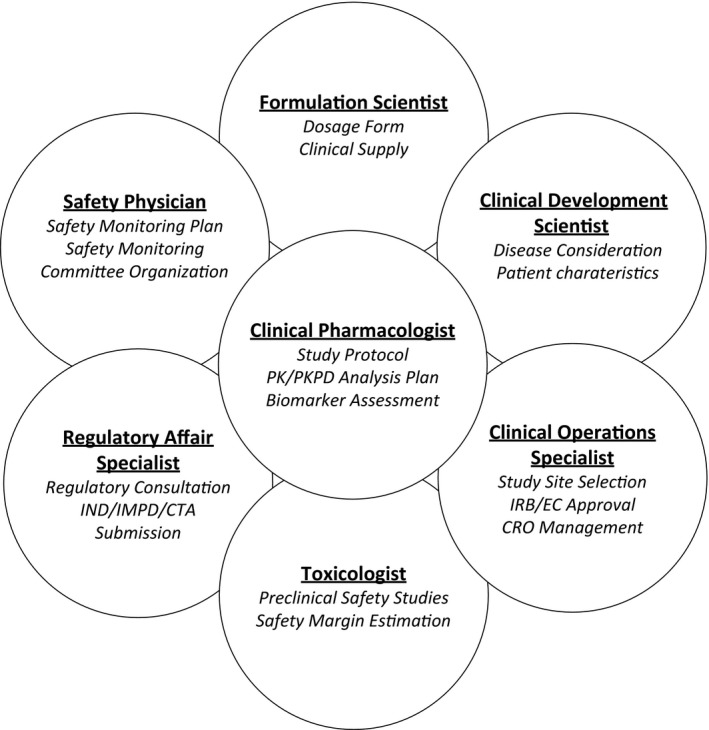
Key stakeholders and their main responsibilities in planning a first‐in‐human study. CTA, clinical trial application; CRO, contract research organization; EC, ethics committee; IMPD, Investigational Medicinal Product Dossier; IND, investigational new drug; IRB, institutional review board; PK, pharmacokinetic; PKPD, pharmacokinetic pharmacodynamic.
