Abstract
Background:
Multiparametric flow cytometry (MFC) is a useful tool for diagnosis of plasma cell dyscrasias and assessment of minimal residual disease (MRD) in plasma cell myeloma (PCM). However, the immunophenotypic differences between the clonal plasma cells (PCs) of plasma cell myeloma (PCM) and those of monoclonal gammopathy of undetermined significance (MGUS) as well as the correlation of these flow cytometric markers with pertinent laboratory parameters have not been evaluated.
Methods:
We retrospectively identified all newly diagnosed treatment-naive PCM and MGUS patients between 09/2014 and 06/2015 who underwent 10-color flow-cytometric evaluation: CD45, CD38, CD138, cKappa, cLambda, CD19, CD27, CD28, CD56, CD117. FACSDiva analysis was utilized to identify antigenic aberrancies and associations with pertinent laboratory parameters were evaluated.
Results:
All cases demonstrated at least 2 aberrancies. There was a trend toward a greater number of aberrancies in PCM, with 68% showing >/= 4 aberrancies compared to 44% in MGUS (p=0.11). The only marker more frequently aberrant in one disease class was CD19, aberrant in 68% of PCM and 25% of MGUS (p<0.01). In PCM, significant associations were found for CD56 non-aberrancy (p=0.05) and the presence of amyloid and CD27 aberrancy and normal serum albumin (p=0.05). In MGUS, CD117 expression was associated with normal hemoglobin (p=0.03).
Conclusions:
The plasma cells of PCM show a trend toward more antigenic aberrancy than those of MGUS. There is significant association between the antigenic profiles of PCM/MGUS and clinical parameters including amyloidosis, albumin level, and hemoglobin.
Keywords: plasma cell myeloma, monoclonal gammopathy, immunophenotyping, flow cytometry
Introduction:
Plasma cell myeloma (PCM) is the third most common hematologic malignancy in the US comprising slightly more than 15% of all hematologic malignancies(1). It accounts for approximately 1.8% of all cancers with an age-adjusted incidence of six per 100,000 per year(2). Clonal plasma cell proliferative disorders(3, 4) encompass a spectrum ranging from an asymptomatic pre-malignant stage termed monoclonal gammopathy of undetermined significance (MGUS)(5) to an intermediate clinical stage of smoldering myeloma to symptomatic PCM. The diagnosis of MGUS relies on finding either serum monoclonal protein of <30 g/L or a clonal bone marrow plasma cell population of <10% in a patient with otherwise no features of end organ damage (CRAB symptoms: C= hypercalcemia, serum calcium >11mg/dl, R= renal insufficiency, creatinine clearance <40ml/min, A= anemia, hemoglobin <10g/dl or B= one or more osteolytic lesions (each lesion >= 5mm in size on X-ray, CT or PET-CT) attributable to plasma cell proliferation. The rate of progression of MGUS to PCM is 0.5–1% per year, but the precise risk is affected by the concentration of the monoclonal protein, type of monoclonal protein, serum free light chain ratio, bone marrow plasmacytosis, proportion of phenotypically clonal plasma cells (PCs), and presence of immunoparesis, defined as reduction of one to two non-involved immunoglobulin isotype levels(5, 6, 7, 8, 9, 10, 11). Based on the revised International Myeloma Working Group (IMWG) Diagnostic criteria, the diagnosis of smoldering PCM requires serum monoclonal protein of >30g/L, and/or clonal bone marrow plasma cells >=10% without any of the myeloma-defining end organ damage (CRAB) symptoms attributable to the neoplastic plasma cell proliferation. Symptomatic myeloma requires presence of CRAB symptoms (together with an M protein or clonal plasma cell proliferation at any level). Additionally the following new biomarkers when present in smoldering myeloma patients have been shown to be predictive of symptomatic myeloma: clonal bone marrow PCs of >60%, involved: uninvolved serum free light chain ratio >/=100 or >/= 1 focal lesions on MRI imaging (IMWG)(5).
With highly specific flow cytometric markers (or combination of markers) allowing unequivocal identification of PCs and characterization of aberrant PC phenotypes enabling discrimination between the normal and clonal PC populations, MFC is useful in diagnosis and assessment of MRD in PCM. In light of recent updates to the diagnostic criteria and the importance of achieving stringent complete remission (sCR) as defined by IMWG (12, 13), the immunophenotypic evaluation of neoplastic PCs using MFC is gaining in popularity. sCR includes normal serum free light chain ratios and absence of clonal PCs in bone marrow by immunohistochemistry or immunofluorescence at a sensitivity level of 10−3 (12) in addition to the criteria required for complete response: negative immunofixation in serum and urine, disappearance of any soft tissue plasmacytomas and <5% PCs in bone marrow. In addition to flow cytometry, immunoglobulin (Ig) allele-specific oligonucleotide-based quantitative polymerase chain reaction (ASO-PCR), next generation sequencing of Ig genes and newer imaging modalities like positron emission tomography (PET) are a few other techniques being utilized for MRD detection in PCM. Paiva et al have recently reviewed these various techniques in MRD assessment in PCM (13). Briefly, some of the advantages of flow cytometry over other techniques includes greater specificity and sensitivity (detection of one tumor cell among 10,000 bone marrow (BM) cells), intra-assay quality check of the whole cell sample via simultaneous detection of hematopoietic populations (B-cells, granulocytes etc), faster results and wide availability at acceptable costs (13, 14, 15, 16). The need for extensive expertise to analyze flow cytometric data, together with the lack of well-standardized flow-MRD methods, have been pointed out as the main drawbacks of flow cytometry (13).
Many studies have investigated a wide range of markers to better define the immunophenotypic profile of aberrant PCs with a few studies looking at the prognostic impact of those markers. In our study, using single-tube 10-color flow cytometry, we were interested in evaluating the utility of various markers in distinguishing clonal PCs of PCM versus those of MGUS with the aim of better defining the immunophenotype of neoplastic PCs, as well as to evaluate the relationship between immunophenotypic markers and disease characteristics.
Materials and Methods:
Patients:
For the purpose of this study all PCM patients (n=22) newly diagnosed between 09/2014 and 06/2015 who underwent 10-color flow cytometric evaluation at the time of initial diagnosis were retrospectively identified using the laboratory information system. Patients with new or previous diagnosis of treatment-naive MGUS (n=16) who underwent 10-color flow cytometric evaluation during the same time period were also similarly identified. MFC data from BM aspirates of each of these patients, obtained as part of routine clinical work up was reviewed as detailed below. Additionally, information on various clinical parameters at the time of diagnosis including level of serum M protein, serum free light chain ratio, levels of hemoglogin, calcium, serum albumin, beta-2 microglobulin, lactate dehydrogenase and creatinine, and the presence of amyloid and light chain cast nephropathy were also collected. This study was approved by the Institutional Review Board of the University of Iowa Hospitals and Clinics.
Flow cytometric immunophenotyping:
Immunophenotyping studies were carried out on the bone marrow aspirates using pre-titrated volumes of the following monoclonal antibodies: CD19 Peridinin-chlorophyll proteins-cyanine 5.5 (Per CP-Cy ™5.5), CD45 Violet 500 (V500), CD117 Allophycocyanin (APC), CD56 APC R700, CD38 Brilliant ™ Violet 605 (BV605), CD138 Brilliant ™ Violet 421 (BV421), CD28 Phycoerythrin Cy7 (PE-Cy™7) and CD27 APC-H7[BD Biosciences, San Jose, CA], and polyclonal λ fluorescein isothiocyanate (FITC) and polyclonal κ PE [Dako Agilent Pathology Solutions, Santa Clara, CA]. Briefly, following red blood cell lysis, the samples were resuspended in RPMI and 2% fetal calf serum and the cell suspension was adjusted to get a concentration of 1 ×10 6 cells/ml. For staining of surface antibodies 100 μL of cell suspension was mixed with the appropriate concentration of monoclonal antibodies and brilliant stain buffer and incubated for appropriate times as per manufacturer’s recommendation. At the end of incubation period the cells were washed with Hank’s balanced salt solution and centrifuged. Cytoplasmic staining for κ and λ was done with Invitrogen Reagent A and B (Carlsbad, CA) as per manufacturer’s recommendation. Acquisition was done on a flow cytometer (BD FACS Calibur or BD FACS Canto, BD Biosciences), and at least 105 events were acquired in each tube. Analyses were carried out using FACSDiva immunophenotyping software (BD Biosciences).
Flow cytometric data analysis:
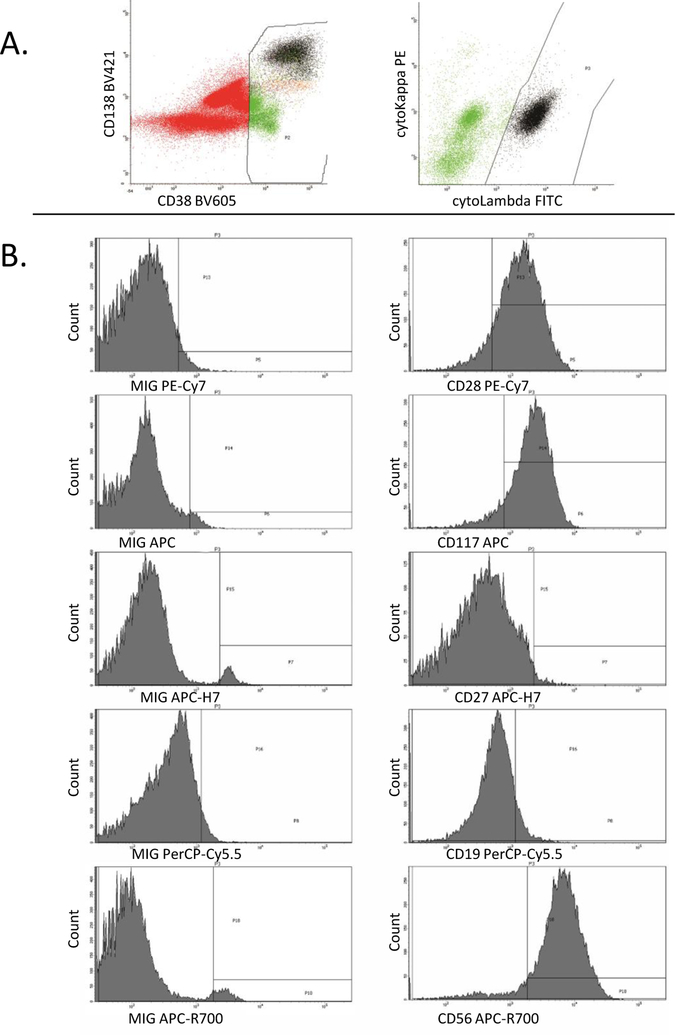
Using FACSDiva analysis, CD38/CD45/CD138/cKappa/cLambda were used to identify the clonal and non-clonal plasma cells populations as described below. All events with comparable or dim CD45 expression compared to lymphocytes were gated on the CD45 vs side scatter histogram. The CD38 vs CD138 histogram was then used to identify the plasma cell population among these gated events based on their bright CD38/CD138 expression. Clonal and non-clonal plasma cell populations were then selected based on the light chain immunoglobulin expression (Figure 1A). Flow cytometric evaluation of the following antigens was done in all cases: CD19, CD27, CD28, CD38, CD45, CD56, CD117, CD138, intracytoplasmic kappa and lambda. The percentage positive for each of the above markers (except CD38 and CD45) was identified after setting the MIG control population at 2–4% positive (Figure 1B). A delta percentage positive was then calculated for each of the markers by subtracting the percentage positive in the non-clonal population from the percentage positive in the clonal population. Aberrant gain (CD28, CD56, CD117, and CD138) and loss (CD19, CD27) of antigen expression was arbitrarily defined as a variation of 10% or more in the delta percentage. This numerical assessment for aberrant antigen expression was an accurate representation of visual inspection of the dot plots. However, due to the arbitrary cut-off of 10%, cases which on visual inspection showed partial dim expression were sometimes categorized as “positive” and sometimes categorized as “negative” by the numerical assessment.
Figure 1:
Example of FACSDiva flow cytometric analysis - bone marrow specimen from a patient with PCM. A. Gating strategy. The cells of interest are identified by bright CD38 expression (left histogram) and cytoplasmic light chain restriction (right histogram). In the MIG (isotype control) tube, cells of interest are identified by bright CD38 expression only. B. Data Analysis. The left column is data from the MIG tube. The right column is data from the antibody-specific tube. The MIG for each fluorochrome is compared to the specific antibody for that fluorochrome. The percentage positive for each of the specific antibodies was identified after setting the MIG control population threshold at 2–4% positive.
Statistical analysis:
All associations between antigen aberrancy and either disease type (PCM OR MGUS) or laboratory parameters were calculated using the chi-square test or Fisher’s exact test for small samples where appropriate. Statistical testing was performed at the 5% level of significance using SAS v9.4 (SAS Institute, Cary, NC)
Results:
There were 16 males and 6 females (age range: 37–76 y; mean: 63) in the PCM cohort. The MGUS cohort had 9 males and 7 females (age range: 45–82 y; mean: 66). The median age was similar in both groups and while there was a male predominance in PCM group (male to female ratio of 2.6:1) compared to MGUS group (male to female ratio of 1.28:1), this difference was not significant (p=0.29). The PCM group included 13 patients with IgG myeloma, 4 patients with IgA myeloma, 4 patients with light chain only myeloma, and 1 patient with non-secretory myeloma. The MGUS group included 12 patients with IgG MGUS and 4 patients with IgA MGUS.
Percentage of non-clonal plasma cells
Immunophenotypically normal PCs (non-clonal PCs) were identified in all the patients of both PCM and MGUS groups. No patient was excluded due to inability to identify non-clonal PCs. The mean percentage of non-clonal PCs in the MGUS group was 17.57 +/−13.79 (range: 0.2 – 42.6%; median: 12.46), which was significantly higher (p value of <0.01) than that of PCM group which had a mean percentage of 4.48 +/− 9.66 (range: 0.002–41.3%; median: 0.43). 81.3% of MGUS patients (13/16) had more than 3% non-clonal PCs, as compared to 31.8% of PCM patients (7/22). This difference was statistically significant (p=0.003).
Antigenic aberrancy differences between MGUS and PCM
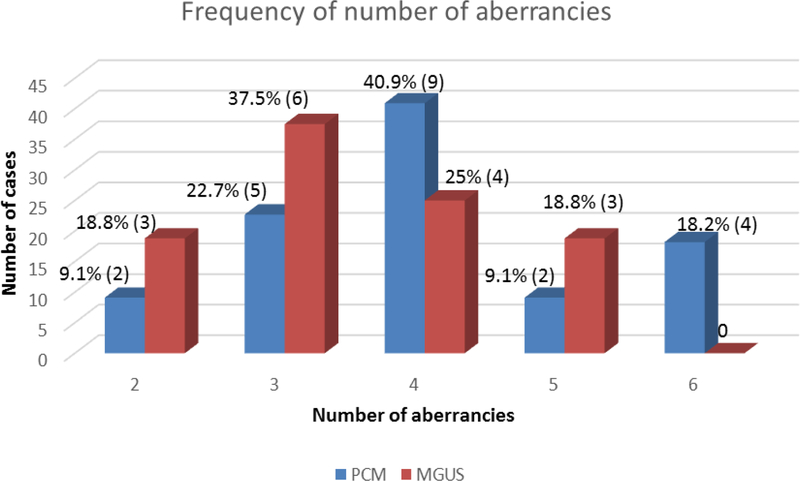
The frequency of aberrant expression of each of the markers was compared between the two groups as illustrated in Table 1. Aberrant expression (loss) of CD19 was found to be significantly more frequent among PCM than MGUS (p= <0.01). Though not achieving statistical significance (p=0.27), we did find a trend toward more frequent CD56-positivity in PCM (82%) compared to MGUS (63%). Next we compared the number of antigenic aberrancies between the two groups (Figure 2). The plasma cells of PCM patients frequently exhibited 4 or more aberrancies (15/22= 68%), whereas patients with MGUS most commonly showed aberrancy for 3 or fewer antigens (9/16=56%) (p=0.11). We also noted that in both groups aberrant expression of CD56 and CD138 and loss of CD27 were the most frequent set of concurrent aberrancies (MGUS-25% [4/16]; PCM-54% [12/22]).
Table 1:
Frequency of aberrancy in expression of different markers in PCM and MGUS groups
| Antigen | PCM N=22 (% aberrant) | MGUS N=16 (% aberrant) | P-value |
|---|---|---|---|
| CD19 | 15 (68.2) | 4 (25) | <0.01 |
| CD27 | 17 (77.3) | 12 (75) | 1.00 |
| CD28 | 9 (40.9) | 6 (37.5) | 0.83 |
| CD56 | 18 (81.8) | 10 (62.5) | 0.27 |
| CD117 | 12 (54.5) | 10 (62.5) | 0.62 |
| CD138 | 18 (81.8) | 13 (81.3) | 1.00 |
Figure 2:
Frequency of number of antigenic aberrancies in PCM and MGUS groups. The frequency of having a given number of antigenic aberrancies is shown above each bar, both as a percentage of total cases within that group and as number of cases (in parenthesis). There were 22 patients total in the PCM group (blue) and 16 patients total within the MGUS group (red).
Correlation between antigen expression and clinical parameters
We also studied the relationship between patterns of antigen expression and laboratory parameters/disease characteristics in each group. The parameters evaluated in the PCM group included the level of serum M-protein, serum free light chain ratio, beta-2 microglobulin, creatinine, calcium, albumin, lactate dehydrogenase, hemoglobin, presence of amyloid, and presence of cast nephropathy. Patients were tested for amyloid deposition by bone marrow and /or fat pad biopsy with or without renal or other organ biopsy as clinically indicated. Within the myeloma group, aberrancy (loss) of CD27 was significantly associated (p=0.05) with normal to high levels of serum albumin (>/= 3.5 mg/dL). This in turn is a predictor of lower stage and good prognosis according to the ISS for PCM. Similarly, non-aberrancy (non-expression) of CD56 was found to be significantly associated (p=0.05) with the presence of amyloid. The parameters evaluated in the MGUS group included hemoglobin level and serum free light chain ratio. Within the MGUS group, aberrant expression (gain) of CD117 on the neoplastic/clonal PCs showed a statistically significant association (p=0.03) with normal hemoglobin levels (within reference ranges for sex) at the time of diagnosis. Since anemia is one of the ‘CRAB’ criteria for diagnosis of PCM, we further investigated the cause of the anemia in the MGUS patients. There were seven MGUS patients with low hemoglobin (6 males and 1 female). Six out of these seven patients had mild anemia (10.2 g/dL in the female patient and range of 11.1–13.1 g/dL among the male patients). One male patient had significant anemia (7.6 g/dL) which predated the diagnosis of MGUS by several years and was attributed to concomitant diabetic chronic kidney disease and sarcoid splenomegaly.
Discussion:
MFC is an increasingly used technique that can be applied to the identification of PCs among all BM cells, as well as the discrimination of phenotypically abnormal PCs from their normal counterparts (10). The high sensitivity of this technique compared to conventional morphologic and immunohistochemical methods has been established(14). Therefore it comes as no surprise that MRD assessment using MFC is standard-of-care in assessing treatment response in PCM patients (13).
However, it is not advisable to utilize MFC as a standalone diagnostic tool in plasma cell proliferative disorders, as there is significant discrepancy in the quantitation of clonal PCs by MFC compared with conventional morphological evaluation. PC enumeration by morphology serves as an important diagnostic criterion(5). Proposed explanations for the discrepancy between PC enumeration by MFC and morphology include contamination of the MFC sample by peripheral blood, existence of small PC clusters, fragility of PCs during sample preparation and unavailability of a first-pull BM aspirate for flow cytometry(17, 18, 19, 20). Availability of first-pull aspirate samples is likely to improve the diagnostic yield of MFC evaluation(19). However, morphology and IHC remain the gold standard for plasma cell enumeration.
The utility of MFC lies in its ability to distinguish aberrant/clonal PCs from the polyclonal normal PC population. This is critical in the differential diagnosis of unusual cases including reactive plasmacytosis, in assessing risk of disease progression(10) in MGUS and smoldering myeloma patients (10, 21, 22), as well as in predicting progression free and overall survival in PCM patients (18, 21, 23, 24). Distinguishing aberrant PCs from normal PCs is less challenging in cases of terminal PCM where the majority of PCs display an aberrant phenotype, than in cases of MGUS where the aberrant cells are in the minority and are usually overshadowed by the presence of large numbers of normal PCs.
In our study we were able to identify non-clonal PCs in all the patients of both PCM and MGUS groups, though they were present at a significantly lower frequency in the PCM group compared to the MGUS group (mean of 4.48% versus 17.57% respectively; median of 0.43% versus 12.46%). The finding of a greater number of immunophenotypically normal plasma cells in MGUS compared with PCM has been observed by other authors (25). Ocqueteau et al proposed a 3% cut-off value for percent residual immunophenotypically normal PCs that they found useful in distinguishing MGUS cases (which were typically >3%) from PCM cases (which were typically <3%). The number of PCM patients with >3% non-clonal PCs was higher in our study than previously reported. However, the 3% cut-off value for percent non-clonal PCs was still found to be a useful parameter to discriminate PCM from MGUS with 81.3% MGUS cases versus 31.8% of PCM cases showing >3% non-clonal PCs (0.003) in our study.
A variety of immunophenotypic aberrancies have been described in plasma cell dyscrasias. However, there is a lack of consensus with respect to the specific panel of markers to analyze. Using a panel very similar to that recommended by the European Myeloma Network (with the exception of CD20, CD200 and CD81) we found that aberrant CD19 expression was more frequently seen in PCM than MGUS. CD19 is a B-lineage marker valuable in the assessment of monoclonal gammopathies. The intensity of CD19 expression is known to be dim on MGUS PCs when compared to normal PCs, but higher on MGUS PCs than on PCM cells(17, 26, 27). Mahmoud et al (1999) also showed that the enforced expression of CD19 on myeloma cell lines led to growth inhibition and reduced tumorigenicity(28). These findings were supported by Olteanu et al (2008) who found that MGUS cases with higher relative proportion of CD19-positive PCs were associated with less frequent disease progression(29). Based on these observations it has been hypothesized that phenotypic loss of CD19 expression might reflect stage transition or disease progression at the genetic level(17). As a practical point, CD19 loss can serve as a useful tool in identifying a small number of abnormal PCs in patients with monoclonal gammopathies. Cannizzo et al (2010) found that CD19 showed good correlation with the presence of disease using a cut off 61% to distinguish between normal and aberrant PCs(30). Tembhare et al (21) in their study also confirmed that CD19 had the highest sensitivity (100%) for detection of abnormal PCs. However they failed to report any difference in pattern of CD19 expression between PCM and MGUS. Our finding that CD19 is more frequently lost in PCM as compared with MGUS is consistent with myeloma being a more advanced disease state than MGUS and highlights the important role this marker plays in identifying MRD in PCM patients.
The prognostic value of MFC in PCM is still not clearly established (19, 31). Studies have attempted to risk stratify patients based on their antigenic profile assessed by MFC (24). In addition, antigens such as CD45, CD56, CD117, and CD28, have also been proposed as prognostic markers for myeloma(19). However whether MFC immunophenotyping will replace the specific serum and genetic markers as a prognostic tool remains to be determined. Our study did not directly assess the association between the various MFC markers and progression free survival or overall survival, as the period of follow-up for these patients was too short.
However, we did make some interesting observations with respect to associations between MFC marker expression and laboratory parameters which are known to have prognostic value. Similar to the findings of Drach et al (1991) and Van Reit et al (1990), we found very frequent (81.8%) positivity for CD56 in PCM(32, 33). We compared the clinical and pathological characteristics of the CD56-positive and negative sub-groups among the PCM group and found the presence of amyloid protein to be significantly associated (p=0.05) with CD56-negative status (Table 2A). CD56 is an isoform of the neural cell adhesion molecule (NCAM) and is known to mediate cell-cell and cell-matrix interactions (17). Absent CD56 expression could accelerate the process of metastatic spread (17, 34, 35) and is correlated with an aggressive disease (36). Also the presence of amyloid in patients with PCM is known to be an independent adverse prognostic factor (37). However, the association of CD56-negative status with amyloid has not been previously reported. Whether CD56-negative PCM is a distinct entity with a worse prognosis will have to be addressed in larger studies involving greater numbers of patients.
Table 2A:
Correlation of clinical and laboratory parameters with antigen expression (p value) in PCM group
| Clinical variable | Reference Range | Mean | Groups (# patients) | CD19 | CD27 | CD28 | CD56 | CD117 | CD138 |
|---|---|---|---|---|---|---|---|---|---|
| Level of serum M-protein | 1 </=3 g/dL(12); >3 g/dL (9) | 0.66 | 0.6 | 0.37 | 1 | 0.67 | 1 | ||
| Beta-2 microglobulin | 1.1–2.4 mg/L | 9.14 | <3.5(8);3.5-<5.5(6); >/=5.5(6) | 0.58 | <0.01 | 0.61 | 0.07 | 0.87 | 0.24 |
| Creatinine | M- 0.6 – 1.2mg/dL F- 0.5 – 1.0 mg/dL |
4.4 | </= 1.2(14); >1.2(7) | 1 | 1 | 1 | 0.57 | 0.4 | 1 |
| Calcium | 8.6–10.2 mg/dL | 9.6 | </= 10.5(18); >10.5(4) | 1 | 1 | 1 | 1 | 1 | 1 |
| Albumin | 3.4 – 4.8 g/dL | 3.5 | <3.5(12);>/=3.5(9) | 1 | 0.05 | 0.67 | 0.6 | 0.66 | 1 |
| Lactate dehydrogenase | M- 135–225 U/L F- 135–214 U/L |
190.4 | </= 225(13); >225(6) | 0.32 | 0.56 | 0.62 | 0.07 | 1 | 1 |
| Amyloid | N/A | Presence (6); absence (16) | 1 | 0.1 | 0.66 | 0.05 | 0.65 | 1 | |
| Cast nephropathy | N/A | Presence(3); absence(19) | 1 | 1 | 1 | 0.47 | 0.57 | 0.47 | |
| Serum free light chain ratio | 0.26–1.65 (N) | 2normal (1); mildly abnormal (11); markedly abnormal (10) | 0.56 | 0.05 | 1 | 0.66 | 0.52 | 0.07 | |
| Hemoglobin | M- 13.2–17.7 g/dL F- 11.9–15.5 g/dL |
11.6 | 3abnormal (14); normal(8) | 0.34 | 0.31 | 1 | 0.6 | 1 | 0.25 |
One patient did not have level of serum M protein determined.
For patients with a kappa-restricted plasma cell clone, the groups compared are normal (0.26–1.65); mildly abnormal (>1.65 and <100); markedly abnormal (>/=100). For patients with a lambda-restricted plasma cell clone, the groups compared are normal (0.26–1.65); mildly abnormal (>0.01 and < 0.26); markedly abnormal (</= 0.01).
Abnormal hemoglobin is defined as <13.2 g/dL for males and <11.9 g/dL for females.
We found a similar frequency of CD27 loss in the MGUS (75%) and PCM (77.3%) groups. We also noted that loss of CD27 expression was more often associated with simultaneous loss of CD19 (13/17) in PCM compared to MGUS (4/12) (Data not shown). These findings are in agreement with those of Raja et al 2010 who also described lack of CD27 expression to be closely coupled with the loss of CD19 and as a marker of progression to PCM (17). In our study we also noted that loss of CD27 in PCM was significantly associated (p=0.05) with normal to high serum albumin levels (Table 2A), which in turn correlates with a lower Revised International Staging System (RISS) and Durie-Salmon stage. This is a novel finding not yet described in the literature.
We found CD117 positivity on clonal PCs of 54.5% PCM patients and 62.5% MGUS patients. While these figures are slightly higher than those reported by other authors, the trend of greater positivity amongst MGUS patients compared to PCM cohort is well-established (38). Expression of CD117 is known to be associated with favorable genetic mutations and a better outcome in both MGUS and PCM patients (38). For the first time, we showed CD117 expression to be associated with normal hemoglobin levels (p=0.03) at the time of diagnosis in MGUS group (Table 2B). This finding supports Bataille’s et al observation of a subset of prognostically favorable CD117+ MGUS/PCM.
Table 2B:
Correlation of laboratory parameters with antigen expression (p value) in MGUS group
| Clinical variable | Reference Range | Groups | Mean | CD19 | CD27 | CD28 | CD56 | CD117 | CD138 |
|---|---|---|---|---|---|---|---|---|---|
| Hemoglobin | M- 13.2–17.7 g/dL F- 11.9–15.5 g/dL |
abnormal (7); normal (9) | 13.05 | 0.58 | 1 | 0.3 | 1 | 0.03 | 0.58 |
| Serum free light chain ratio | 0.26–1.65 | Normal (11); mildly abnormal (5); markedly abnormal(0) | 0.55 | 1 | 0.09 | 1 | 0.59 | 0.55 |
Finally the trend toward a larger number of aberrancies in PCM as compared to MGUS suggests the possibility of clonal evolution. None of the three MGUS patients with 5 or more aberrancies showed earlier progression to PCM. One of these patients died of decompensated cardiac failure due to an unrelated cause. An exhaustive work up including a fat pad biopsy failed to reveal any evidence of amyloid in this case.
In summary, our retrospective study identified significant, novel associations between the antigen profile of PCM and MGUS plasma cells and several clinical and laboratory parameters which are known to predict prognosis in plasma cell neoplasms. Our analyses showed a correlation between the presence of amyloid and CD56-negative PCM, thereby providing a possible clue for the poor prognosis in this group. In addition our findings of normal albumin levels in CD27-negative PCM and normal hemoglobin levels in CD117+ MGUS have not yet been described. Albumin is known to correlate with stage/prognosis as it is part of the ISS. As multiple comparisons increase the probability of a Type 1 error (false positive association), larger studies are necessary to confirm these findings. We confirmed the diagnostic sensitivity of CD19 in distinguishing between PCM and MGUS (p<0.01) which is in agreement with the findings of other authors. We also found a trend towards greater number of aberrancies with disease progression, thereby suggesting the possibility of a clonal evolution (with at least two aberrancies seen in all plasma cell dyscrasias studied).
Based on the above findings we recommend that a carefully chosen panel of antigens (to include at a minimum CD38, CD138, CD19, CD56, CD27, cKappa, and cLambda) in a single tube can reliably and consistently distinguish clonal plasma cell dyscrasias from reactive plasma cells and holds promising potential for prognostication in MGUS and PCM.
Acknowledgements:
The authors have no conflict of interest to declare. They gratefully acknowledge Sabrina L Bonde, laboratory scientist in the Flow Cytometry Laboratory at the University of Iowa Hospitals and Clinics during the time of this study (currently technical specialist for Becton Dickinson) for her outstanding technical support.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Röllig C, Knop S, Bornhäuser M. Multiple myeloma. The Lancet; 385: 2197–2208. [DOI] [PubMed] [Google Scholar]
- 3.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med 2011; 364: 1046–1060. [DOI] [PubMed] [Google Scholar]
- 4.Rajkumar SV. Treatment of multiple myeloma. Nat Rev Clin Oncol 2011; 8: 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos M-V, Kumar S et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. The Lancet Oncology 2014; 15: e538–e548. [DOI] [PubMed] [Google Scholar]
- 6.Rajkumar SV, Kyle RA, Therneau TM, Melton LJ, Bradwell AR, Clark RJ, Larson DR, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood 2005; 106: 812–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turesson I, Kovalchik SA, Pfeiffer RM, Kristinsson SY, Goldin LR, Drayson MT, Landgren O. Monoclonal gammopathy of undetermined significance and risk of lymphoid and myeloid malignancies: 728 cases followed up to 30 years in Sweden. Blood 2014; 123: 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, Melton LJI. A Long-Term Study of Prognosis in Monoclonal Gammopathy of Undetermined Significance. New England Journal of Medicine 2002; 346: 564–569. [DOI] [PubMed] [Google Scholar]
- 9.Cesana C, Klersy C, Barbarano L, Nosari AM, Crugnola M, Pungolino E, Gargantini L, et al. Prognostic Factors for Malignant Transformation in Monoclonal Gammopathy of Undetermined Significance and Smoldering Multiple Myeloma. Journal of Clinical Oncology 2002; 20: 1625–1634. [DOI] [PubMed] [Google Scholar]
- 10.Pérez-Persona E, Vidriales M-B, Mateo G, García-Sanz R, Mateos M-V, de Coca AG, Galende J, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood 2007; 110: 2586–2592. [DOI] [PubMed] [Google Scholar]
- 11.Katzmann JA, Clark R, Kyle RA, Larson DR, Therneau TM, Melton LJ, Benson JT, et al. Suppression of uninvolved immunoglobulins defined by heavy/light chain pair suppression is a risk factor for progression of MGUS. Leukemia 2013; 27: 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, Gertz M, et al. International uniform response criteria for multiple myeloma. Leukemia 2006; 20: 1467–1473. [DOI] [PubMed] [Google Scholar]
- 13.Paiva B, van Dongen JJM, Orfao A. New criteria for response assessment: role of minimal residual disease in multiple myeloma. Blood 2015; 125: 3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flores-Montero J, de Tute R, Paiva B, Perez JJ, Böttcher S, Wind H, Sanoja L, et al. Immunophenotype of normal vs. myeloma plasma cells: Toward antibody panel specifications for MRD detection in multiple myeloma. Cytometry Part B: Clinical Cytometry 2016; 90: 61–72. [DOI] [PubMed] [Google Scholar]
- 15.Pedreira CE, Costa ES, Lecrevisse Q, van Dongen JJM, Orfao A. Overview of clinical flow cytometry data analysis: recent advances and future challenges. Trends in Biotechnology 2013; 31: 415–425. [DOI] [PubMed] [Google Scholar]
- 16.Szczepanski T, Orfao A, van der Velden VH, San Miguel JF, van Dongen JJ. Minimal residual disease in leukaemia patients. The Lancet Oncology 2001; 2: 409–417. [DOI] [PubMed] [Google Scholar]
- 17.Raja KRM, Kovarova L, Hajek R. Review of phenotypic markers used in flow cytometric analysis of MGUS and MM, and applicability of flow cytometry in other plasma cell disorders. British Journal of Haematology 2010; 149: 334–351. [DOI] [PubMed] [Google Scholar]
- 18.Paiva B, Vidriales M-B, Pérez JJ, Mateo G, Montalbán MA, Mateos MV, Bladé J, et al. Multiparameter flow cytometry quantification of bone marrow plasma cells at diagnosis provides more prognostic information than morphological assessment in myeloma patients. Haematologica 2009; 94: 1599–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rawstron AC, Orfao A, Beksac M, Bezdickova L, Brooimans RA, Bumbea H, Dalva K, et al. Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica 2008; 93: 431–438. [DOI] [PubMed] [Google Scholar]
- 20.Smock KJ, Perkins SL, Bahler DW. Quantitation of Plasma Cells in Bone Marrow Aspirates by Flow Cytometric Analysis Compared With Morphologic Assessment. Archives of Pathology & Laboratory Medicine 2007; 131: 951–955. [DOI] [PubMed] [Google Scholar]
- 21.Tembhare PR, Yuan CM, Venzon D, Braylan R, Korde N, Manasanch E, Zuchlinsky D, et al. Flow cytometric differentiation of abnormal and normal plasma cells in the bone marrow in patients with multiple myeloma and its precursor diseases. Leuk Res 2014; 38: 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pérez-Persona E, Mateo G, García-Sanz R, Mateos M-V, De Las Heras N, De Coca AG, Hernández JM, et al. Risk of progression in smouldering myeloma and monoclonal gammopathies of unknown significance: comparative analysis of the evolution of monoclonal component and multiparameter flow cytometry of bone marrow plasma cells. British Journal of Haematology 2010; 148: 110–114. [DOI] [PubMed] [Google Scholar]
- 23.Paiva B, Vidriales M-B, Mateo G, Pérez JJ, Montalbán MA, Sureda A, Montejano L, et al. The persistence of immunophenotypically normal residual bone marrow plasma cells at diagnosis identifies a good prognostic subgroup of symptomatic multiple myeloma patients. Blood 2009; 114: 4369–4372. [DOI] [PubMed] [Google Scholar]
- 24.Mateo G, Montalbán MA, Vidriales M-B, Lahuerta JJ, Mateos MV, Gutiérrez N, Rosiñol L, et al. Prognostic Value of Immunophenotyping in Multiple Myeloma: A Study by the PETHEMA/GEM Cooperative Study Groups on Patients Uniformly Treated With High-Dose Therapy. Journal of Clinical Oncology 2008; 26: 2737–2744. [DOI] [PubMed] [Google Scholar]
- 25.Ocqueteau M, Orfao A, Almeida J, Blade J, Gonzalez M, Garcia-Sanz R, Lopez-Berges C, et al. Immunophenotypic characterization of plasma cells from monoclonal gammopathy of undetermined significance patients. Implications for the differential diagnosis between MGUS and multiple myeloma. Am J Pathol 1998; 152: 1655–1665. [PMC free article] [PubMed] [Google Scholar]
- 26.Harada H, Kawano M, Huang N, Harada Y, Iwato K, Tanabe O, Tanaka H, et al. Phenotypic difference of normal plasma cells from mature myeloma cells. Blood 1993; 81: 2658–2663. [PubMed] [Google Scholar]
- 27.Zandecki M, Facon T, Bernardi F, Izydorczyk V, Dupond L, François M, Reade R, et al. CD19 and immunophenotype of bone marrow plasma cells in monoclonal gammopathy of undetermined significance. Journal of Clinical Pathology 1995; 48: 548–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahmoud MS, Fujii R, Ishikawa H, Kawano MM. Enforced CD19 Expression Leads to Growth Inhibition and Reduced Tumorigenicity. Blood 1999; 94: 3551–3558. [PubMed] [Google Scholar]
- 29.Olteanu H, Wang H-Y, Chen W, McKenna RW, Karandikar NJ. Immunophenotypic studies of monoclonal gammopathy of undetermined significance. BMC Clinical Pathology 2008; 8: 13–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cannizzo E, Bellio E, Sohani AR, Hasserjian RP, Ferry JA, Dorn ME, Sadowski C, et al. Multiparameter immunophenotyping by flow cytometry in multiple myeloma: The diagnostic utility of defining ranges of normal antigenic expression in comparison to histology. Cytometry Part B: Clinical Cytometry 2010; 78B: 231–238. [DOI] [PubMed] [Google Scholar]
- 31.Mateo Manzanera G, San Miguel Izquierdo JF, Orfao de Matos A. Immunophenotyping of plasma cells in multiple myeloma. Methods in molecular medicine 2005; 113: 5–24. [DOI] [PubMed] [Google Scholar]
- 32.Drach J, Gattringer C, Huber H. Expression of the neural cell adhesion molecule (CD56) by human myeloma cells. Clinical and Experimental Immunology 1991; 83: 418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Camp B, Durie B, Spier C, De Waele M, Van Riet I, Vela E, Frutiger Y, et al. Plasma cells in multiple myeloma express a natural killer cell- associated antigen: CD56 (NKH-1; Leu-19). Blood 1990; 76: 377–382. [PubMed] [Google Scholar]
- 34.Blaheta RA, Beecken W-D, Engl T, Jonas D, Oppermann E, Hundemer M, Doerr HW, et al. Human Cytomegalovirus Infection of Tumor Cells Downregulates NCAM (CD56): A Novel Mechanism for Virus-Induced Tumor Invasiveness. Neoplasia (New York, NY) 2004; 6: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blaheta RA, Hundemer M, Mayer G, Vogel J-U, Kornhuber B, Cinatl J, Markus BH, et al. Expression Level of Neural Cell Adhesion Molecule (NCAM) Inversely Correlates with the Ability of Neuroblastoma Cells to Adhere to Endothelium In Vitro. Cell Communication & Adhesion 2002; 9: 131–147. [DOI] [PubMed] [Google Scholar]
- 36.Sahara N, Takeshita A, Shigeno K, Fujisawa S, Takeshita K, Naito K, Ihara M, et al. Clinicopathological and prognostic characteristics of CD56-negative multiple myeloma. British Journal of Haematology 2002; 117: 882–885. [DOI] [PubMed] [Google Scholar]
- 37.Vela-Ojeda J, García-Ruiz Esparza MA, Padilla-González Y, Sánchez-Cortes E, García-Chávez J, Montiel-Cervantes L, Reyes-Maldonado E, et al. Multiple myeloma-associated amyloidosis is an independent high-risk prognostic factor. Annals of Hematology 2009; 88: 59–66. [DOI] [PubMed] [Google Scholar]
- 38.Bataille R, Pellat-Deceunynck C, Robillard N, Avet-Loiseau H, Harousseau J-L, Moreau P. CD117 (c-kit) is aberrantly expressed in a subset of MGUS and multiple myeloma with unexpectedly good prognosis. Leukemia Research 2008; 32: 379–382. [DOI] [PubMed] [Google Scholar]