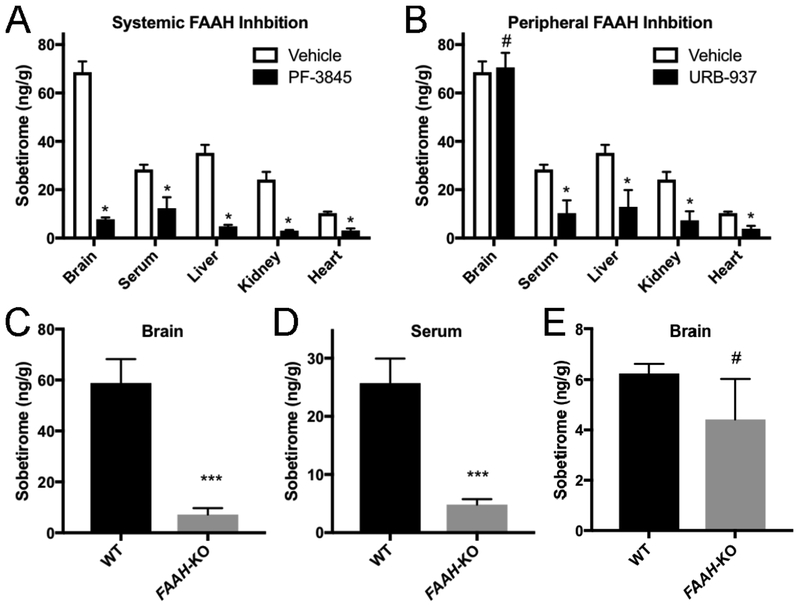
Figure 3. FAAH is the primary hydrolase responsible for sobetiramide hydrolysis in vivo.
(A & B) FAAH inhibitors significantly decrease Sob-AM2 hydrolysis in target tissues. Mouse cohorts (n=3) were treated with vehicle, PF-3845, or URB-937 (i.p., 1 mg/kg). 30 min post initial injection, mice were treated with Sob-AM2 (i.p., 3.05 μmol/kg). Tissues were collected 1 h after the second injection. (A) Consistent with its known pharmacological distribution, PF-3845 significantly reduced sobetirome levels in all tested tissues compared with vehicle. (B) Compared to the vehicle control, URB-937 reduced sobetirome levels in all tested tissues except for the brain, consistent with its inability to cross the BBB. Data for vehicle treated animals is common to (A) and (B) and is replicated for clarity. Data represent mean ± SEM and statistical analyses were performed using multiple t-test comparing inhibitor treatment to vehicle. (C-E). Wild type and FAAH-KO mice were treated with Sob-AM2 (i.p., 3.05 μmol/kg) and tissues were collected 1 h post-dose. Sobetirome levels analyzed by LC-MS/MS. FAAH-KO mice exhibited significant lowering of sobetirome in both the brain (C) and serum (D) compared to wild type animals that receiving an identical systemic dose. (E) Following a peripheral dose of the parent drug sobetirome (i.p., 3.05 μmol/kg), FAAH-KO mice showed no significant difference in brain sobetirome levels compared with wild type. Data represent mean ± SEM. (C) n=6; (D) and (E) n=3. Statistical analyses were performed using two-tailed Student t-tests (***P < 0.001, # P> 0.05)