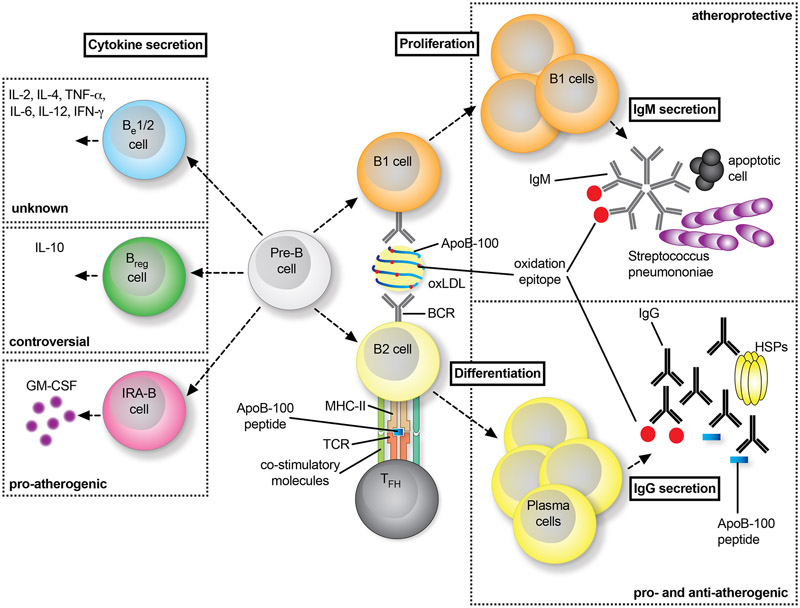
Figure 4: Distinct role of B cells in atherosclerosis.
B cells on a developmental stage (Pre-B) turn into innate-like B1 cells or adaptive, conventional B2 cells (right panel). B1 cells recognize epitopes on LDL and oxLDL particles, which leads to their activation and expression of low-affinity IgM antibodies by proliferation. Often, these IgM show cross-reactivity with epitopes on bacteria such as Streptococcus pneumoniae or on apoptotic cells. Interfering with B1 functionality aggravates atherosclerosis. B2 cells require co-stimulation by TFH cells by MHC-II:peptide:TCR interactions and co-stimulatory signaling events to fully differentiate into plasma cells that express high-affinity IgG antibodies against atherogenic antigens, such as ApoB, oxLDL, or heat-shock proteins (HSP). Neutralizing B2 cells is atheroprotective, while the role of IgG-antibodies remains controversial with reported pro- and anti-atherogenic functions. Independent of the classification of B1/2 cells, distinct B cell subsets have been shown to express non-exclusive sets of cytokines, which allows the definition of cytokine-secreting B-effector (Be) −1 and −2 cells, regulatory B cells (Bregs), and innate-response activator (IRA) B cells (left panel).