Figure 5. Differentiation characteristics for human neural stem cells (hNSCs) and mesenchymal stem cells (hMSCs) embedded in the 3D ECM created by the BMA-EDMA pattern and comparison with 2D differentiation.
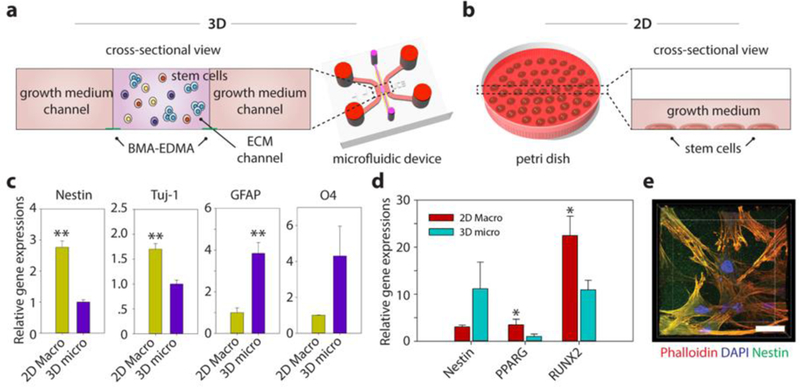
a. Schematic cross-sectional view of the 3D stem cell differentiation assay. A 3D cellular microenvironment was created by placing a mixture of stem cells and pre-gel solution of MAT (final cell density: 2 million cells/mL) within the BMA-EDMA pattern. Both growth medium channels were filled with the appropriate growth media supplemented with growth factors to maintain stemness. b. Illustration depicting 2D stem cell culture in a conventional cell culture dish, for comparison. The normal level for growth media in a culture dish/flask is approximately 1–2 mm. c. Relative gene expressions of hNSCs (on day 3) for Nestin (neural stem cell), neuron-specific Class III ß-tubulin (Tuj-1; neuron), glial fibrillary acidic protein (GFAP; astrocyte), and O4 (oligodendrocyte) in 3D micro (a) and 2D macro (b). Data are shown as the means ± SD (n = 3). **p<0.01. d. Relative gene expressions of hMSCs (on day 3) for Nestin, PPARG (adipocyte), and Runt-related transcription factors 2 (RUNX2; osteocyte) in 3D micro and 2D macro conditions. The differentiation fate of hMSCs was found to be highly dependent on the culture dimension/geometry. Cells cultured in 3D showed lower expression of RUNX2, indicating that the 3D microenvironmental MAT effectively maintained their stemness, while unwanted osteocyte differentiation could not be inhibited in 2D culture. Data are shown as the means ± SD (n = 3). p<0.05. e. A confocal microscope image shows the hMSCs cultured within MAT in the BMA-EDMA device. Actin filaments and nuclei of hMSCs were immunostained with rhodamine Phalloidin (red) and DAPI, respectively. Nestin expression (green) was monitored using a rabbit anti-Nestin antibody and goat anti-Rabbit Alexa Fluor 488 antibody. Scale bar: 20 µm. All error bars: one standard deviation.
