Fig. 2.
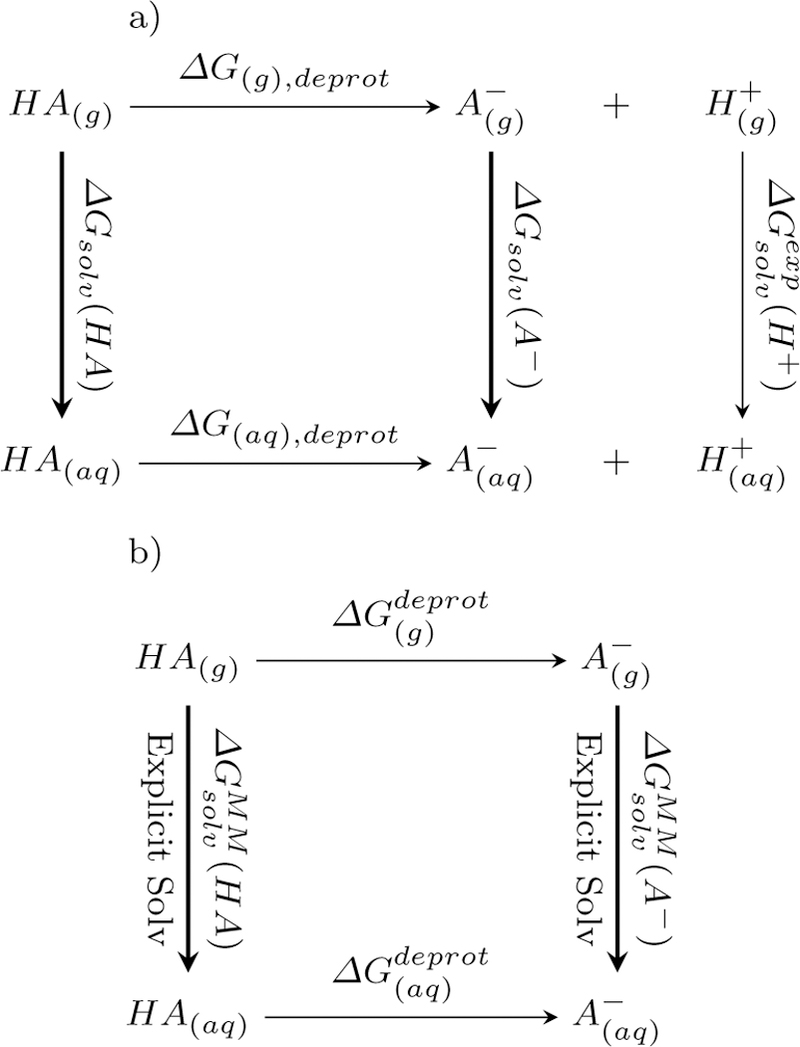
Thermodynamic cycles used in the pKa calculations a) chemical reaction of acid dissociation. This relates the free energy of dissociation in the aqueous phhase as with the gas phase free energy of dissociation and solvation free energies of the acid, base and proton. b) Alchemical cycle for deprotonation. This cycle relates the solavtion free energy difference of the HA and A− with difference in free energy for deprotonation in the aqueous and gas phases.
