Abstract
Objective
The purpose of this study was to design and implement a realistic, durable, and low-cost training model for percutaneous renal access.
Material and methods
Ballistic gelatin mixed with radiographic contrast was poured into surgical gloves to create a radio-dense renal collecting system. The collecting system model was then embedded in a pure ballistic gelatin block resting upon a clear acrylic glass base. Finally, the model was covered by a visually opaque polyurethane foam cover with chalk sticks positioned to simulate ribs. Experienced attending urologists and interventional radiologists, urology residents, and medical students used the model to access the upper, middle, and lower renal calyces under fluoroscopic guidance. Outcomes included model durability, realism rated by participants on a visual analogue scale, and cost.
Results
The ballistic gelatin model was durable and anatomically realistic. Each model sustained over 200 needle punctures with no significant compromise in structural integrity or any contrast leakage. Attending and resident physicians considered it to provide an accurate simulation of renal access and medical students and residents considered the model to be a practical training modality (residents 8.4/10 vs. medical students 9.4/10). The total cost for one model was $60.
Conclusion
The ballistic gelatin collecting system provided a realistic, durable, and low-cost renal access training model. This could allow trainees to develop skills without compromising patient safety.
Keywords: Education, percutaneous nephrolithotomy, surgical model
Introduction
Obtaining renal access during percutaneous nephrolithotomy (PCNL) represents one of the most challenging steps of the surgery. The risk of bleeding, pneumothorax, hydrothorax, and injury to adjacent organs during percutaneous renal access can make acquisition of skills daunting for urology residents and potentially hazardous for the patient. Direct training in the operating room (OR) can also add a significant amount of time and cost to surgical cases.[1] In an attempt to facilitate the acquisition of surgical skills, various training models for PCNL have been described.[2–8] However, these models are frequently limited by not being realistic, lack of durability, and high cost.[2–8]
In an attempt to address these limitations, a novel model was created using ballistic gelatin. Ballistic gelatin uses animal collagen to simulate human tissue and is commonly used to assess the effects of penetrating and blast injuries on soft tissue.[9] However, its use beyond the evaluation of traumatic injury and application to medical training has been limited. The purpose of this study was to design and implement a realistic, durable, and low-cost kidney model using ballistic gelatin for percutaneous renal access that could improve a novice surgeon’s technical skills without compromising patient safety.
Material and methods
The initial step in model construction was to design a radiopaque collecting system that would enable fluoroscopic-guided access while also being durable allowing repeated needle accesses without rupture of the collecting system. After experimenting with a variety of methods, it was discovered that an appropriate mixture of ballistic gelatin and contrast would create a realistic collecting system that could be easily visualized under low-dose fluoroscopy. A mixture of 10% ballistic gelatin was combined with 30% iohexol containing contrast material (Omnipaque 300, GE Healthcare, Princeton, NJ, USA) and 60% water by weight and poured into a small nitrile examination glove (Halyard Health, Inc., Alpharetta, GA, USA) fashioned into the shape of a renal collecting system. Calyceal length was adjusted by tying off the fingers of the glove with a diameter of 1.5–2 cm. Infundibular width was adjusted using half-inch electrical tape. The contrast-enhanced-ballistic gelatin was allowed to set at 2 degrees Celsius for 3 hours to create a model of a radio-dense renal collecting system.
Next, the radiopaque collecting system was placed into a 24 × 13 × 6.5 cm rectangular mold and covered with 10% ballistic gelatin and allowed to set for an additional 12 hours at 2 degrees Celsius. The finished gelatin block containing a renal collecting system (Figure 1) was then removed from the mold and placed onto a clear acrylic base. Layers of thick, visually opaque polyurethane foam (Premium Poly Foam, American Excelsior Company, Arlington, TX) were used to encase and cover the gelatin block to limit visualization and mimic skin, fat, and muscle overlying the kidney. Chalk sticks measuring 10 × 1.5 × 1.5 cm were embedded between layers of the Poly Foam cover to simulate the density and firmness of ribs (Figure 2).
Figure 1.
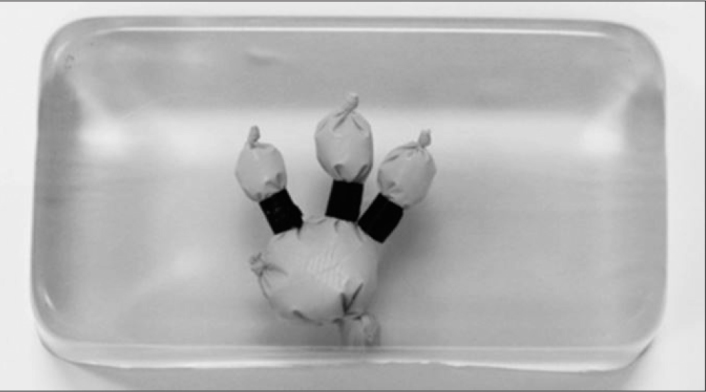
Ballistic gelatin block with radio-opaque renal collecting system model composed of a surgical glove fashioned into the shape of the renal collecting system and filled with ballistic gelatin enhanced with contrast
Figure 2.
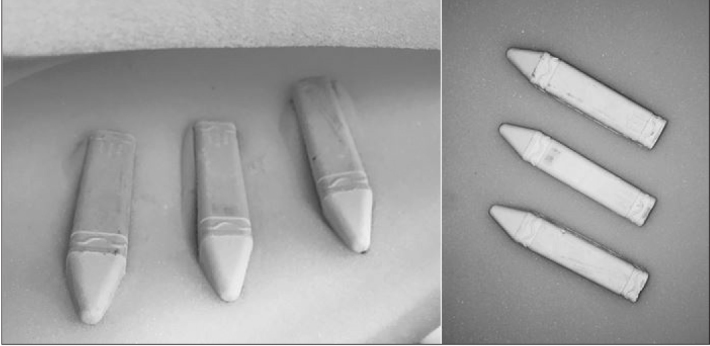
Chalk sticks embedded within the Poly Foam cover to simulate ribs 10–12
After creation of the model the durability and validity of the model was tested in a simulation of percutaneous renal access using fluoroscopic guidance (Figure 3). Attending urologists and interventional radiologists, urology residents, and medical students used the model to gain needle access into the upper, middle, and lower renal calyces. Successful puncture was confirmed by direct visualization through the clear acrylic glass base or from visualization through the side of the gelatin block. For training purposes, participants were assessed and provided feedback through the number of punctures and course corrections made prior to obtaining renal access as well as total fluoroscopy time.
Figure 3.
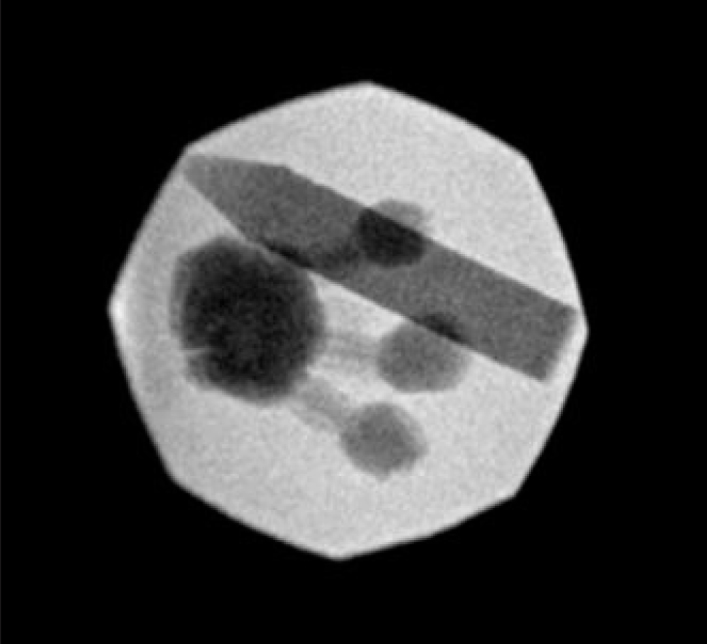
Fluoroscopic appearance of the ballistic gelatin model with simulated rib
Endpoints included model realism, durability, and cost. An anonymous survey of attending physicians, residents, and students was also used to determine whether the model was representative of renal collecting system anatomy and provided an accurate simulation of the complexity of gaining percutaneous access. To evaluate the content validity of this training model in simulating percutaneous renal access, participants used a visual analogue scale (1–10) to rate the adequacy of calyceal visualization, interference from chalk ribs, depth of the collecting system, and resistance of the Poly Foam cover and ballistic gelatin in comparison with human tissue.
Results
The ballistic gelatin model was accommodated to upper, middle, and lower calyceal access by 6 attending physicians, 6 residents, and 10 medical students. A single model sustained over 200 punctures with no significant compromise in structural integrity. Although ballistic gelatin should be refrigerated for long-term storage, the model was resistant to manipulation with no compromise to its integrity despite being stored at room temperature for 12 hours. Furthermore, contrary to the traditional training models, the contrast-enhanced gelatin did not leak when punctured by a needle. The model remained intact for the entire duration of a 1-month study period.
Survey results for medical students, urology residents, attending physicians, and all participants are listed in Table 1. Fluoroscopic calyceal visualization (medical students 9.1, residents 9.8, attendings 9.8) and obstruction from simulated chalk ribs (medical students 8.4, residents 9.0, attendings 9.5) were rated highly by all participants. However, the depth of the collecting system was not rated as highly by residents (residents 7.8 vs. attendings 9.2) and the similarity between gelatin and real tissue was moderately rated (residents 7.0 vs attendings 6.8). Ultimately attending and resident physicians considered the model to provide an accurate simulation of the renal collecting system (residents 8.3 vs. attendings 8.4). Medical student participants were unable to determine model realism on the survey, but both medical students and residents considered the model to be a practical training modality that would improve their ability to obtain renal access (medical students 9.4 vs. residents 8.5).
Table 1.
Subjective evaluation of the ballistic gelatin model as an accurate simulation of percutaneous renal access on a visual analogue scale of 1–10 with 10 being the highest rating. Scores are presented as mean (± standard deviation)
| Students | Residents | Attendings | All participants | |
|---|---|---|---|---|
| Could you identify the renal calyces on fluoroscopy? | 9.1 (1.4) | 9.8 (0.4) | 9.8 (0.4) | 9.5 (1.1) |
| Did the chalk rib block your needle access and did you have to adjust your technique because of the presence of pieces of chalk? | 8.4 (2.8) | 9.0 (1.2) | 9.5 (1.0) | 8.8 (2.1) |
| Was the depth of the collecting system appropriate? | n/a | 7.8 (1.0) | 9.2 (1.3) | 8.8 (1.2) |
| Did the needle puncturing the gel feel like real tissue? | n/a | 7.0 (1.4) | 6.8 (2.3) | 7.4 (1.7) |
| Did the model provide an accurate simulation of a real renal collecting system? | n/a | 8.3 (1.0) | 8.4 (1.8) | 8.5 (1.3) |
| Did working with the model mimic renal access? | n/a | 8.0 (0.7) | 7.4 (2.2) | 7.7 (1.4) |
| Do you feel that your ability to obtain renal access would be improved using this training model? | 9.4 (1.0) | 8.4 (0.5) | 7.6 (2.4) | 8.7 (1.5) |
The total cost of materials to create 5 models was $300 with one model costing $60. The retail price for a 4.5 kg bag of ballistic gelatin was $130, the cost of 100 ml of contrast material was $70 at our institution, and a large roll of Poly Foam was $70. There were insignificant costs from chalk, electric tape, and gloves.
Discussion
Obtaining renal access for PCNL is challenging and associated with a significant risk of complications. The complication rate of PCNL ranges from 12.5%[10] to 30.3%.[11] Many of the concerning complications, such as pneumothorax, hydrothorax, and injury to adjacent organs are related to percutaneous renal access. Since interventional radiologists are not always available and not all urologists practice in tertiary care centers, it is therefore important for urologists to maintain and improve their skills of gaining renal access. As surgeons that understand the size, length, and angle of the infundibulum and the preferable location of a percutaneous renal tract, urologists have a critical understanding of what constitutes optimal renal access. Despite similar levels of technical difficulty, there have been less access-related complications and greater stone-free rates in patients whose renal access was achieved by urologists compared to interventional radiologists.[12]
There is nevertheless a learning curve for the technical skills needed to perform PCNL. It has been reported that a urologist needs to complete 60 PCNLs to achieve surgical competence and 115 cases to reduce fluoroscopy time and radiation to levels equivalent to those of a senior surgeon.[13,14] Training in residency can influence the ability to gain one’s own PCN access and perform PCNL in clinical practice.[15] Of the urologists that were trained in obtaining percutaneous renal access in residency, 27% continue to achieve PCN access by themselves and 92% of them perform percutaneous surgeries.[15] In contrast, 11% of urologists that were not trained to realize renal access in residency perform their own PCN intrarenal access in clinical practice and only 33% of them perform percutaneous surgeries.[15]
There has been a pedagogical shift away from learning surgical skills solely in the OR. Although surgical training in the OR is still necessary, training models can provide a more approachable learning curve that allows resident physicians to learn and develop fundamental skills without compromising patient safety. Not only are training models an effective and inexpensive means of improving the technical skills of surgical residents,[16] they also allow for guided instruction and independent practice, which can improve their performance and laparoscopic skills.[17,18] The technical skills acquired from bench-top models and simulations have been shown to transfer to technical skills when working with human cadavers.[19] Within the field of urology, benchtop models of the genitourinary system have been effective in developing endourologic skills.[20]
Training models have been specifically used for gaining renal access and performing PCNL. Animal models primarily describe using porcine kidneys based on structural similarities with human kidneys.[21] Strohmaier and Giese[2] reported the use of an en bloc porcine kidney model. In addition to the kidney and ureter, retroperitoneal organs were harvested en bloc from freshly slaughtered pigs to create an ex vivo training model.[2] The collecting systems of these kidneys were opened to incorporate calculi prior to use for percutaneous endourologic procedures. Earp[3] described the addition of a foam layer on top of the porcine kidney to simulate the resistance of human tissue. This inexpensive kidney model allowed for percutaneous maneuvers and renal access using fluoroscopy.[3]
More complex animal models have incorporated the use of chicken carcasses. A porcine-chicken carcass model was first reported by Hammond et al.[4] Porcine kidneys with intact ureters from commercially slaughtered pigs were instilled with pebbles to simulate nephrolithiasis. These kidneys were then placed into eviscerated chicken carcasses purchased from the supermarket. Ureteral catheters were used to inject contrast into the collecting system and needle access, tract dilation, and renal access sheath insertion were performed under fluoroscopy. This model also allowed for nephroscopy, grasper use, and stone fragmentation.[4] Each model was reusable for 3 procedures. This model was modified by Häcker et al.[5] to incorporate ultrasound (US) and renal perfusion. The chicken carcass was filled with US gel in order to enable the use of US during renal access, and a medical perfusion pump was used to perfuse the kidney with heparinized blood or saline to maintain tissue tension.[5] Following renal access and tract dilation, the artificial stone was extracted using a nephroscope and lithotriptor.
Technological advancements have recently introduced the use of customized non-animal kidney models. Bruyere et al.[6] presented a case report in which a prototype of a patient’s anatomy was created prior to PCNL. Computer-aided design used radiographic images to make virtual cross-sections of the patient’s kidney. These cross-sections were then used to create a laminated model. An attending urologist and trainee performed a simulated PCNL on the laminated model prior to completing an uneventful PCNL on the patient. Bruyere et al.[6] suggested that prototype models may reduce the morbidity of PCNLs by providing urologists with simulated training before the actual surgery, especially in patients with complex renal anatomy. The reported cost of this model was $3690.[6]
Customized models have also become available through technological advancements with three-dimensional (3D) printing. Turney[7] printed training models for percutaneous renal access by extracting collecting system anatomy from reformatted computed tomography images. These models, composed of a water-soluble polyvinyl alcohol plastic, were embedded in silicone to create a mold of a collecting system. The 3D model was dissolved and irrigated out of the silicone before being replaced by contrast medium. The silicone model was then covered with a layer of foam to replicate tissue and provide training for percutaneous access under fluoroscopic guidance. Turney[7] reported that using 3D printing could create different models that could account for variations in the anatomy of the human collecting system.[7] This model was only able to sustain 20 punctures prior to contrast leakage. While the cost of each model was quoted to be $100 per model, the additional costs for software and a 3D printer were $9600 and $3200, respectively.[7] This model is nevertheless cheaper than virtual reality trainers and artificial organ models.
In our study, the total cost of materials to create 5 ballistic gelatin models was approximately $300, which amounted to $60 per model. This is significantly less expensive than laminated prototypes[6] and 3D printing.[7] When factoring in the number of punctures sustained in a single ballistic gelatin model, the cost is comparable with purchasing multiple porcine kidneys and chicken carcasses. Not only is the model cost-effective, but it provides training that could reduce operative time and, therefore, operative costs.[1]
A similar cost-effective model used latex gloves filled with contrast media and then covered with foam.[8] However, this model did not adjust the shape of the gloves to represent collecting system anatomy. This model can also be limited by leakage of contrast material following glove puncture. In our model, the combination of ballistic gelatin with iohexol contrast formed a solidified renal collecting system that did not leak, a potential limitation of other collecting system models.[7,8] This enabled the model to sustain over 200 needle punctures without loss of integrity.
The ballistic gelatin model is also clean without the risk of parasitic or bacterial contamination compared to using porcine kidneys and chicken carcasses. The ballistic gelatin model can be stored for an extended period of time using refrigeration and does not require special storage or safe handling. It can be kept at room temperature for up to 12 hours without concerns for odor or juices that can come from raw animal organs or carcasses. Working with the gelatin model subsequently does not require additional covers, gowns, or masks to protect trainees from contamination. Furthermore, porcine kidneys require processing to cleanse the kidney of blood clots and chicken carcasses require evisceration. The modified model by Hacker et al. also describes the use of perfusion to maintain tissue tension,[5] which would require additional equipment that may not be readily available.
While the ribs simulated by chalk sticks added additional complexity to this training model, this model can be modified in multiple ways to increase difficulty. The size of the dilated calyces and renal pelvis in our training model was purposely enlarged to facilitate training. Variations can be made to decrease the size of the calyces and the degree of hydronephrosis to create a more challenging model. Examination gloves can also be manipulated and shaped to represent different anatomic anomalies, such as horseshoe kidneys, calyceal diverticula, and malrotated kidneys. The ballistic gelatin block also allows for the incorporation of other anatomic models, such as the diaphragm, colon, and other adjacent organs, for additional complexity. The thickness of the Poly Foam cover can be varied by adding additional layers to simulate obesity. The ballistic gelatin model may have potential application to other medical specialties, such as interventional radiology.
There are some limitations to our study. One limitation is that this training model was limited to gaining percutaneous renal access but did not incorporate tract dilation, nephroscopy, or lithotripsy of stones. However, achieving optimal renal access is one of the most challenging aspects of PCNL. Although most urologists feel comfortable with stone removal, only a minority are comfortable obtaining their own access. For this reason, needle access is the most important step to emphasize in a training model. Another limitation of our model is that the ballistic gelatin model does not incorporate the respiratory excursion seen in a normal patient during surgery. We did not feel that this significantly limited training since percutaneous access can be achieved during end-expiration when the kidney is not moving. Another limitation is that this model may not be optimal for learning US-guided access as the Poly Foam cover contains a significant amount of air, which can obscure visualization of the calyces on US. However, removal of the Poly Foam layer could also allow this model to be used during training for US-guided access. Despite these limitations, the ballistic gelatin model provides a realistic training model for repetitive training at a very reasonable cost.
In conclusion, ballistic gelatin can be used to create a realistic, durable, and low-cost renal access training model. This model allows the development of technical skills in a low stress environment without compromising patient’s safety. Use of realistic training models could shorten the learning curve for urologic surgeons to realize their own percutaneous renal access.
Footnotes
Ethics Committee Approval: This study was investigating a bench top model and subsiquently no IRB was required. There were no human subjects in this study.
Informed Consent: This was a bench top model and there were no human subjects involved in the research, thus no informed consent was necessary.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - J.M.E., M.C.W., S.M.E.; Design - J.M.E., M.C.W., S.M.E.; Supervision - D.D.B., M.H., J.W.C., J.M.E.; Materials - J.M.E., M.C.W., S.M.E.; Data Collection and/or Processing - J.M.E., J.W.C., S.M.E., M.C.W., H.W.; Analysis and/or Interpretation - J.W.C., H.W., M.H., D.D.B.; Literature Search - J.W.C., H.W., J.M.E.; Writing Manuscript - J.W.C., H.W., J.M.E.; Critical Review - J.M.E., J.W.C., H.W., M.H., D.D.B.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors have declared that they did not have any financial support for this study.
References
- 1.Bridges M, Diamond DL. The financial impact of teaching surgical residents in the operating room. Am J Surg. 1999;177:28–32. doi: 10.1016/S0002-9610(98)00289-X. [DOI] [PubMed] [Google Scholar]
- 2.Strohmaier WL, Giese A. Improved ex vivo training model for percutaneous renal surgery. Urol Res. 2009;37:107–10. doi: 10.1007/s00240-009-0180-x. [DOI] [PubMed] [Google Scholar]
- 3.Earp PP. Percutaneous renal surgery: new model for learning and training. Int Braz J Urol. 2003;29:151–4. doi: 10.1590/S1677-55382003000200011. [DOI] [PubMed] [Google Scholar]
- 4.Hammond L, Ketchum J, Schwartz BF. A new approach to urology training: a laboratory model for percutaneous nephrolithotomy. J Urol. 2004;172:1950–2. doi: 10.1097/01.ju.0000140279.15186.20. [DOI] [PubMed] [Google Scholar]
- 5.Häcker A, Wendt-Nordahl G, Honeck P, Michel MS, Alken P, Knoll T. A biological model to teach percutaneous nephrolithotomy technique with ultrasound-and fluoroscopy-guided access. J Endourol. 2007;21:545–50. doi: 10.1089/end.2006.0327. [DOI] [PubMed] [Google Scholar]
- 6.Bruyere F, Leroux C, Brunereau L, Lermusiaux P. Rapid prototyping model for percutaneous nephrolithotomy training. J Endourol. 2008;22:91–6. doi: 10.1089/end.2007.0025. [DOI] [PubMed] [Google Scholar]
- 7.Turney BW. A new model with an anatomically accurate human renal collecting system for training in fluoroscopy-guided percutaneous nephrolithotomy access. J Endourol. 2014;28:360–3. doi: 10.1089/end.2013.0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lezrek M, Tazi H, Slimani A, Sadiq A, Fethi A, Bazine K, et al. A cheaper simulator for learning percutaneous renal access: a latex glove model. Videourology. 2013;27:5. doi: 10.1089/vid.2013.0023. [DOI] [Google Scholar]
- 9.Cronin DS. Material properties for numerical simulations for human, ballistic soap and gelatin. Defence R&D Canada-Valcartier; 2010. [Google Scholar]
- 10.de la Rosette JJ, Opondo D, Daels FP, Giusti G, Serrano A, Kandasami SV, et al. Gius. Categorisation of complications and validation of the Clavien score for percutaneous nephrolithotomy. Eur Urol. 2012;62:246–55. doi: 10.1016/j.eururo.2012.03.055. [DOI] [PubMed] [Google Scholar]
- 11.Mousavi-Bahar SH, Mehrabi S, Moslemi MK. Percutaneous nephrolithotomy complications in 671 consecutive patients: a single-center experience. Urol J. 2011;8:271–6. [PubMed] [Google Scholar]
- 12.Watterson JD, Soon S, Jana K. Access related complications during percutaneous nephrolithotomy: urology versus radiology at a single academic institution. J Urol. 2006;176:142–5. doi: 10.1016/S0022-5347(06)00489-7. [DOI] [PubMed] [Google Scholar]
- 13.Tanriverdi O, Boylu U, Kendirci M, Kadihasanoglu M, Horasanli K, Miroglu C. The learning curve in the training of percutaneous nephrolithotomy. Eur Urol. 2007;52:206–12. doi: 10.1016/j.eururo.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Allen D, O’Brien T, Tiptaft R, Glass J. Defining the learning curve for percutaneous nephrolithotomy. J Endourol. 2005;19:279–82. doi: 10.1089/end.2005.19.279. [DOI] [PubMed] [Google Scholar]
- 15.Lee CL, Anderson JK, Monga M. Residency training in percutaneous renal access: does it affect urological practice? J Urol. 2004;171:592–5. doi: 10.1097/01.ju.0000104849.25168.6d. [DOI] [PubMed] [Google Scholar]
- 16.Wanzel KR, Matsumoto ED, Hamstra SJ, Anastakis DJ. Teaching technical skills: training on a simple, inexpensive, and portable model. Plast Reconstr Surg. 2002;109:258–64. doi: 10.1097/00006534-200201000-00041. [DOI] [PubMed] [Google Scholar]
- 17.Powers TW, Murayama KM, Toyama M, Murphy S, Denham EW, 3rd, Derossis AM, et al. Housestaff performance is improved by participation in a laparoscopic skills curriculum. Am J Surg. 2002;184:626–9. doi: 10.1016/S0002-9610(02)01096-6. [DOI] [PubMed] [Google Scholar]
- 18.Coleman RL, Muller CY. Effects of a laboratory-based skills curriculum on laparoscopic proficiency: a randomized trial. Am J Obstet Gynecol. 2002;186:836–42. doi: 10.1067/mob.2002.121254. [DOI] [PubMed] [Google Scholar]
- 19.Anastakis DJ, Regehr G, Reznick RK, Cusimano M, Murnaghan J, Brown M, et al. Assessment of technical skills transfer from the bench training model to the human model. Am J Surg. 1999;177:167–70. doi: 10.1016/S0002-9610(98)00327-4. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto ED, Hamstra SJ, Radomski SB, Cusimano MD. A novel approach to endourological training: training at the Surgical Skills Center. J Urol. 2001;166:1261–6. doi: 10.1097/00005392-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Sampaio FJ, Pereira-Sampaio MA, Favorito LA. The pig kidney as an endourologic model: Anatomic contribution. J Endourol. 1998;12:45–50. doi: 10.1089/end.1998.12.45. [DOI] [PubMed] [Google Scholar]


