Abstract
1,25-Dihydroxyvitamin D3 (1,25(OH)2D) elicits a transcriptional response in the intestines. Assessments of this response are often derived from crude tissue homogenates and eliminate the ability to discriminate among different cell types. Here, we used an RNA in situ hybridization assay, RN Scope (Advanced Cell Diagnostics, Newark, CA), to identify the cells in the intestine that respond to 1,25(OH)2D with expression of cytochrome P450 family 24 subfamily A member 1 (Cyp24a1) mRNA. Mice were gavaged with a single bolus dose of 1,25(OH)2D to target the duodenum or a glucuronic acid conjugate of 1,25(OH)2D, β-G-1,25(OH)2D, to target the colon. QRT-PCR analysis of Cyp24a1 mRNA verified that the 1,25(OH)2D-induced responses were present. RNAScope revealed that the mRNA response present after six hours is limited to mature enterocytes exposed to the intestinal lumen in both the duodenum and colon. No detectable expression was observed in goblet cells, lamina propria, muscularis mucosa muscle, submucosa and submucosal lymphoid follicles, or tunica muscularis. Our findings have identified epithelial enterocytes to be the intestinal targets for 1,25(OH)2Dinboththe duodenum and colon.
Keywords: Vitamin D; 1,25-Dihydroxyvitamin D; Calcitriol; Duodenum; Colon
1. Introduction
1,25-Dihydroxyvitamin D3 (1,25(OH) 2D) is the active form of vitamin D3 that binds to the vitamin D receptor (VDR) and initiates events that modulate gene expression. These events lead to transcriptional upregulation of calcium transport proteins as well as proteins involved in apoptosis, cell differentiation, and inflammation [1, 2]. In the intestines, 1,25(OH)2D increases the uptake of dietary calcium, but the localization of the mRNA response within the tissue architecture has not been confirmed.
In the mouse, both the duodenum and colon respond to orally administered 1,25(OH)2D [1]. The colon, however, requires larger doses to surpass the small intestine. These larger doses of 1,25(OH)2D inadvertently perturb systemic 1,25(OH)2 and may cause deleterious effects. To eliminate potential complications, our laboratory previously synthesized a therapeutic glucuronide of 1,25(OH)2D, β-glucuronic-1,25(OH)2D (β-G-1,25(OH)2D) [3]. Conjugation with a glucuronic acid moiety via beta-linkage renders the β-G-1,25(OH)2D molecule inaccessible to mammalian digestive enzymes. When ingested, the glucuronide moiety remains intact through the small intestine until arriving in the colon. There bacterial glucuronidases in the colon hydrolyze the beta-linked acid and liberate 1,25(OH)2D. Thus, β-G-1,25(OH)2D pharmacologically delivers 1,25(OH)2D to the colon for hormonal action [3, 4].
Activation of VDR by 1,25(OH)2D-binding induces expression of an array of genes containing vitamin D response elements (VDREs) [5]. One particular gene, cytochrome P450 family 24 subfamily A member 1 (Cyp24a1), encodes for the 24-hydroxlase that initiates degradation of 1,25(OH)2D in a negative feedback manner [6]. The promoter region of this gene contains two VD Es and, consequently, is robust and sensitive to the actions of ligand-bound VDR [7]. Thus, increased abundance of Cyp24a1 mRNA, as assessed by quantitative real time PCR (QRT-PCR), is utilized as an index of tissue responsiveness to 1,25(OH)2D. Unfortunately, this method involves RNA extraction from crude tissue homogenization and does not allow for determination of the individual cells responding to 1,25(OH)2D within the tissue.
Previous immunohistochemistries of VDR and 1,25(OH)2D-induced proteins indicate that the Strongest response in the intestines occurs in the epithelial layer, but confirmation of expression at the transcriptional level has not yet been demonstrated [8, 9, 10, 11]. Recent advances in RNA in situ hybridization (ISH) have enabled chromogenic detection of individual mRNA targets in tissue sections. In the RNAScope assay by Advanced Cell Diagnostics (Newark, CA), hybridizations between modified DNA probes and the complementing target mRNA undergo an amplification that enhances sensitivity with minimal background detection [12]. In the following study, we use RNAScope to assess the induction of yp24a1 mRNA by 1,25(OH)2D in both the duodenum and colon of mice to confirm the hypothesis that the 1,25(OH)2D-response occurs in the epithelial cells of the intestine.
2. Methods
2.1. Animals
Twenty-one adult male CD1 mice were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were housed in groups and fed a standard diet (Teklad 2018, Madison, WI) ad libitum, with unlimited access to food and water in a 12-hour light/dark cycle. Rodent care and handling procedures were approved by the Iowa State University Institutional Animal Care and Use Committee.
2.2. Animal experiments
1,25-Dihydroxyvitamin D3 was purchased from Sigma Aldrich (St. Louis, MO),and β-G-1,25(OH)2D was synthesized by methods previously described [3]. Compounds were dissolved in 100% ethanol and stored at −86° C. Concentrations of 1,25(OH)2D and β-G-1,25(OH)2D were quantified by using the molar extinction coefficient of 18,300 −1cm−1 at 264 nm absorbance. Oral treatments of ethanol, 1,25(OH)2D, or β-G-1,25(OH)2D were diluted with peanut oil to a volume of 50 μL and gavaged in a single dose. Three mice were gavaged with ethanol in peanut oil to serve as 0 pmol controls. Treatment groups included nine mice each and were further divided into three doses: 6 pmol, 12 pmol, or 24 pmol (n=3 mice per dose per treatment). Six hours after treatment, mice were anesthetized by inhalation of isoflurane and euthanized by guillotine. Blood was collected from the cervical stump into heparinized tubes. Blood plasma samples were stored at −20° C and used for 1,25(OH)2D analysis by radioimmunoassay performed by Heartland Assays (Ames, IA). Two 1-cm segments were collected from the duodenum and proximal colon of mice. Intestinal segments were flushed with ice-cold 0.9% sodium chloride solution. One segment from each tissue was homogenized in 1 mL TRIzol Reagent (Invitrogen, Carlsbad, CA) and stored at −86° C until RNA extraction. The other segment was fixed with 10% formalin in neutral phosphate buffer for 24 hours, then immediately embedded in paraffin for RNA ISH.
2.3. RNA extraction
Total RNA was extracted from TRIzol homogenates. RNA was solubilized by using the recommended chloroform separation per TRIzol reagent protocol (Invitrogen, Carlsbad, CA). The upper aqueous phase was removed and subjected to RNeasy mini prep column (Qiagen, Germantown, MD). Total RNA extraction was performed as recommended with an added wash (2 M NaCl and 2 mM EDTA at pH 4.0) as previously described [4]. Columns were eluted with 50 μL nuclease-free water and the total RNA concentration determined by using the absorbance at 260 nm wavelength. RNA quality was verified with 260:280 nm of approximately 2.0. Samples were diluted to 0.5μg total RNA/uL of nuclease-free water. cDNA was synthesized from 1 μg of RNA by using random hexamer primers and SuperScript III First Strand Synthesis (Invitrogen, Carlsbad, CA). cDNA samples were diluted to a final volume of 100 μL with Tris-EDTA buffer (10 mM Tris and 1 mM EDTA) and stored at −20° for analysis by QRT-PCR.
2.4. QRT-PCR analysis
QRT-PCR was performed on an Mx3005p thermal cycler by Stratagene (La Jolla, CA). cDNA was amplified with PerfeCTa SYBR Green FastMix Low Rox (Quanta Biosciences, Inc, Gaithersburg, MD) for 45 cycles of melting (96° C, 3 s) and annealing (54° C, 30 s). Primers were synthesized by Integrated DNA Technologies (Coralville, IA) and include murine Cyp24a1 [5’ CACACGCTGGCCTGGGACAC (forward) and 5’ GGAGCTCCGTGACAGCAGCG (reverse)], and murine Gapdh [5’ GAAGGTCGGTGTGAACGGATTTGGC (forward) and 5’ TTGATGTTAGTGGGGTC CGC CCTG (reverse)]. QRT-PCR data are expressed as Ct of Cyp24a1 relative to the Ct of Gapdh (dCt) and normalized to the average dCt of control animals (ddCt).
2.5. RNAScope in situ hybridization
Paraffin-embedded tissues were cut to 5 μm sections by using a rotary microtome. Immediately following sectioning, RNA ISH was completed per RNAScope 2.0 HD Red Manual Detection Kit protocol with pretreatment optimization (Advanced Cell Diagnostics, Newark, CA). Positive (Mus peptidylprolyl isomerase B) and negative (E. coli dihydrodipicolinate reductase) oligo probes were used as procedural controls. Oligo probes for murine Cyp24a1 were custom designed and are proprietary to Advanced Cell Diagnostics. Hybridization of probes to the mRNA and amplification of the hybridizations were performed per protocol and detected by using Fast Red chromogenic dye with hematoxylin counterstain of the tissue. mRNA signal particles were counted by using ImageJ software (US NIH, Besthesda, MD) with a Colour Deconvolution plug-in. Particle signal was counted if the particle size was within 4 to 50 pixels in diameter and at least 0.8 circularity. Particle count was determined as the average of five fields from each section observed at 40× magnification.
2.6. Statistical analysis
Data were analyzed by using the GLM procedure for ANOVA comparisons with Tukey-Kramer adjustments for multiple comparisons and the REG procedure for correlations in SAS statistical software (SAS Institute Inc, Cary, NC).
3. Results
3.1. Appearance of 1,25(OH)2D into blood plasma
Plasm1,25(OH)2Dconcentrationswere assessed for control mice and mice that received 24 pmol of 1,25(OH)2D or β-G-1,25(OH)2D (Fig. 1). Six hours after treatment, plasma [1,25(OH)2D] were significantly greater for the 1,25(OH)2D-treated animals (559 ± 82 pmol/L) than for control animals (160 ± 17 pmol/L, P < 0.01). An equimolar dose of β-G-1,25(OH)2D also yielded significantly greater concentrations of plasma 1,25(OH)2D (416 ± 52 pmol/L) than for control animals (P < 0.05). Although numerically different, there was no statistical difference between 1,25(OH)2D and β-G-1,25(OH)2D treatments (p = 0.4). The observation of equimolar 1,25(OH)2D and β-G-1,25(OH)2D doses causing similar increases in plasma [1,25(OH)2D] verifies successful hydrolysis of the glucuronic acid moiety in β-G-1,25(OH)2D and the subsequent internalization of liberated 1,25(OH)2D.
Fig. 1. Both1,25(OH)2D and β-G-1,25(OH)2D oral treatments increase plasma [1,25(OH)2D].
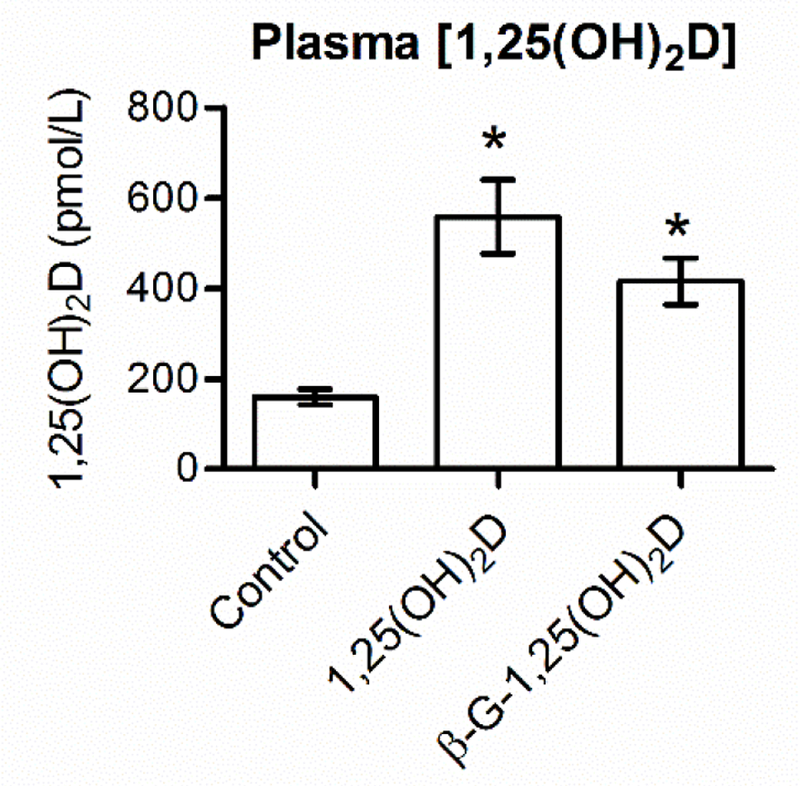
Bars depict mean ± standard error of the mean. *Statistically different from control ( <0.05). 1,25(OH)2D, 1,25-dihydroxyvitamin D3; β-G-1,25(OH)2D, β-glucuronic-1,25-dihydroxyvitamin D3.
3.2. QRT-PCR from crude tissue homogenates
QRT-PCR analysis of Cyp24a1 mRNA was used to quantify the intestinal response to 1,25(OH)2D. In the duodenum, all doses of 1,25(OH)2D (6 pmol, 12 pmol, and 24 pmol) had significantly greater abundances of Cyp24a1 mRNA than controls (P< 0.02; Fig. 2A). The responses followed a dose-dependent relationship in that the effect of the lowest dose (6 pmol, 4.5 ± 0.5 ddCt) was statistically different from the effect of the highest dose (24 pmol, 8.0 ± 0.3 ddCt), and no differences were observed between adjacent increments. In the colon, only the 24 pmol 1,25(OH)2D dose was enough to elicit a small, but statistically significant increase in Cyp24a1 expression compared with the control colons (3.3 ± 0.9 ddCt, P< 0.05; Fig. 2B).
Fig. 2. The mRNA response to 1,25(OH)2D occurs primarily in the duodenum, whereas the response to β-G-1,25(OH)2D occurs in the colon.
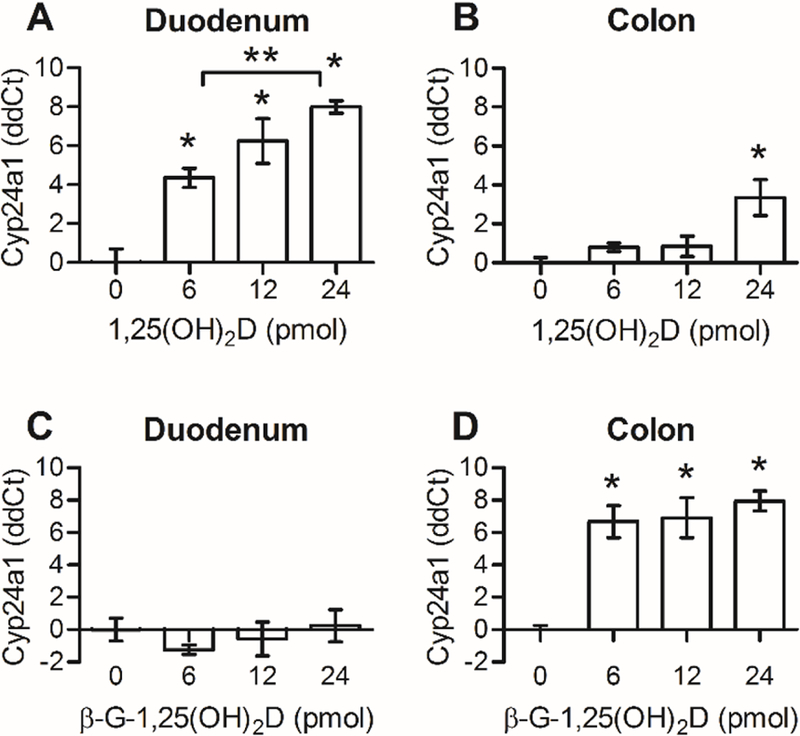
Cyp24a1 ddCt data are calculated as Ct normalized to Gapdh and relative to the average of controls. Bars depict mean ± standard error of the mean. *Statistically different from control with 0 pmol dose (P<0.05). **Statistically different between groups (P<0.05). 1,25(OH)2D, 1,25-dihydroxyvitamin D3; β-G-1,25(OH)2D, β-glucuronic-1,25-dihydroxyvitamin D3; Cyp24a1, cytochrome P450 family 24 subfamily A member 1; Gapdh, glyceraldehyde-3-phosphate dehydrogenase.
Use of β-G-1,25(OH)2D enabled targeting of 1,25(OH)2D to the colon of mice. Mice were treated with β-G-1,25(OH)2D3 in equimolar increments to 1,25(OH)2D dosages. No increase in Cyp24a1 mRNA expression was observed in the duodenum, despite a 2.6-fold increase in plasma [1,25(OH)2D] (Fig. 2C). In the colons, the effects of all β-G-1,25(OH)2D doses were statistically greater than those of controls (P< 0.05), but not different from one another (Fig. 2D), suggesting a maximal threshold was met with 6 pmol β-G-1,25(OH)2D. The lack of duodenal stimulation by β-G-1,25(OH)2D indicates that the glucuronide-bound complex delivers no hormonal stimulation and further supports the assumption that β-G-1,25(OH)2D is biologically inactive until glucuronide hydrolysis by bacterial enzymes [3,4].
3.3. RNAScope in situ hybridization of 1,25(OH)2D-stimulated intestines
RNAScope hybridizations were performed with mus Cyp24a1 probes to locate 1,25(OH)2D-dependent responses in duodenal and colon tissues. This assay amplifies the mRNA:probe hybridizations in tissue sections for visualization under light microscopy. Cyp24a1 mRNA was observed in all 1,25(OH)2D-treated duodena and β-G-1,25(OH)2D-treated colons, whereas the duodena and colons from control animals had no visible expression (Fig. 3). Increases in the dose of 1,25(OH)2D or β-G-1,25(OH)2D increased the density of hybridizations until reaching saturation such that individual mRNA signals could not be distinguished. In both the duodenum and colon, Cyp24a1 mRNA transcripts were present only in epithelial enterocytes. Additionally, Cyp24a1 mRNA was more concentrated in mature enterocytes nearest the interior of the intestinal lumen rather than in enterocytes deep in the crypt regions. Goblet cells, lamina propria cells, submucosa cells, submucosal lymph follicles, and smooth muscle tissue were devoid of Cyp24a1 mRNA transcripts.
Fig. 3. The 1,25(OH)2D-mediated Cyp24a1 mRNA response appears in epithelial enterocytes in both the duodenum and colon.
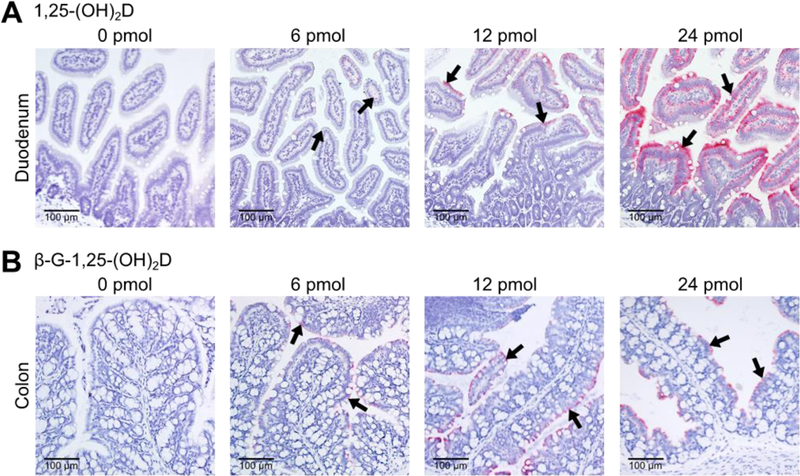
Representative images obtained from RNAScope hybridization with Cyp24a1 probes (indicated by black arrows) at 20× magnification. 1,25(OH)2D, 1,25-dihydroxyvitamin D3; β-G-1,25(OH)2D, β-glucuronic-1,25-dihydroxyvitamin D3; Cyp24a1, cytochrome P450 family 24 subfamily A member 1.
Quantitative analysis of the RNAScope hybridization was performed in ImageJ software. he images were filtered by using the Colour Deconvolution plug-in to isolate the Fast Red chromogenic color. Once filtered, a particle count was performed as described in section 2.5. The 24 pmol dose of 1,25(OH)2D yielded an average particle count for the duodenum that was significantly greater than for the control duodenum, but not statistically different among other doses of 1,25(OH)2D. No statistical differences were observed in the colons across all doses of β-G-1,25(OH)2D, despite significant increases observed by QRT-PCR (Fig. 4B). An inability to distinguish individual mRNA signals at high abundance may have interfered with particle count determination and caused a high degree of variation in the measurement. Variation in particle count may also be affected by the random selection of fields obtained, the sensitivity of the optics and analysis software, and/or the orientation of the tissue when paraffin-embedded. We then compared the RNAScope Cyp24a1 particle count with the corresponding QRT-PCR Cyp24a1 mRNA ddCt for each animal (Fig. 4C, D). By using Pearson’s R (ρ) statistical method, we observed significant positive correlations between QRT-PCR and particle count for both tissues (P < 0.05).
Fig. 4. Particle count analysis of RNA in situ hybridization and its correlation to QRT-PCR data.
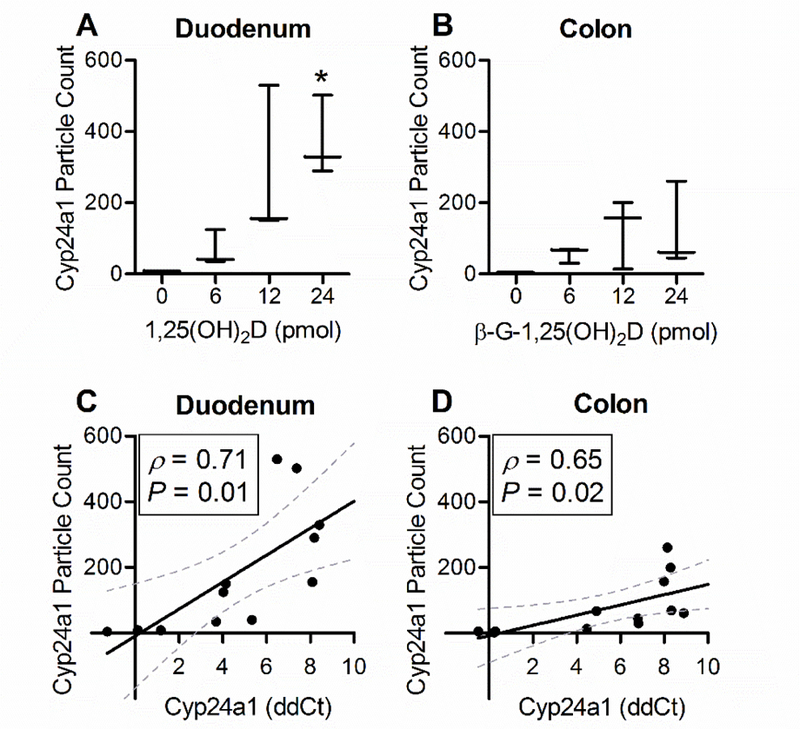
Horizonal lines depict means and whiskers represent minimum and maximum (A, B). Error intervals depict 95% confidence (C, D). *Statistically different from 0 pmol controls (P<0.05). Statistical significance of xy correlations ( ) determined by Pearson’s R analysis (ρ). 1,25(OH)2D, 1,25-dihydroxyvitamin D3; β-G-1,25(OH)2D, β-glucuronic-1,25-dihydroxyvitamin D3; Cyp24a1, cytochrome P450 family 24 subfamily member 1.
4. Discussion
As a steroid hormone, 1,25(OH)2D targets cells in the intestine to increase the mRNA expression of proteins vital for adequate calcium absorption. Through the use of RNAScope ISH, we observed that the 1,25(OH)2D-mediated mRNA response is distinctly limited to enterocytes in both the duodenum and colon of mice 6 hours after treatment (Fig. 3). Furthermore, the mRNA expression is most abundant in enterocytes of the duodenal villi and colonic tips rather than in crypt regions. These findings are consistent with published literature on the1,25(OH)2D-mediated response that used immunohistochemistry. Multiple assessments of intestinal VDR protein had predicted that the response would occur in the enterocyte layer [8, 9, 10, 11]. Wang et al. and Sidler-Lauff et al. additionally noted an absence of VDR in accessory cells such as secretory, muscle and lymphatic cells, corresponding with our mRNA data [10, 11]. Interestingly, Colston et al. and Sidler-Lauff et al. observed the greatest density of VDR in crypt enterocytes rather than in the villi or tips of the intestinal architecture as we have shown. Both researchers also demonstrated the appearance of vitamin D-stimulated proteins alkaline phosphatase and calbindin D9K, respectively, throughout the epithelial layer. These stimulated proteins were most abundant in the villi rather than in crypts, precisely corresponding with our findings [10, 13]. Taken together, the presence of VDR, the transcription of 1,25(OH)2D-stimulated mRNA, and the translation of 1,25(OH)2D-stimulated proteins all agree that the 1,25(OH)2D-mediated response occurs in intestinal epithelial cells at villous or tip regions.
Our validation assessments revealed contradictions between circulating 1,25(OH)2D and the intestinal responses. We found that even when β-G-1,25(OH)2D caused a 2.6-fold increase in plasma [1,25(OH)2D], the expected response in the duodenum was absent. Similarly, when plasma [1,25(OH)2D] increased 3.5-fold from 1,25(OH)2D-treatment, the response in the colon was blunted and could have instead resulted from digestive passage of 1,25(OH)2D. This is a curious finding as current theories propose that 1,25(OH)2D, released from the kidney, circulates in the blood to reach the duodenal cells by presumably crossing the basolateral membrane of enterocytes [1, 13]. Our data suggest that the duodenum, and possibly the colon, may not respond directly to fluctuations in circulating 1,25(OH)2D. A similar phenomenon had been demonstrated in the mouse by Koszewski et al. [4]. They found that the colon responded to subcutaneous administration of both 1,25(OH)2D and β-G-1,25(OH)2D treatments, but these responses were abolished when the intestine of the mouse was surgically tied off below the bile duct to prevent passage of bile and digesta. The data collected by Koszewski et al. suggested that the colon receives circulating 1,25(OH)2D and/or β-G-1,25(OH)2D via bile delivery to the apical membrane rather than via circulation to the basolateral membrane. Our finding that the location of the response is only in the epithelial cells further supports the notion of bile delivery of 1,25(OH)2D into the intestine. While endogenous 1,25(OH)2D and β-G-1,25(OH)2D have not yet been observed in bile, a glucuronide of 25-hydroxyvitamin D3, the precursor to 1,25(OH)2D, has been shown in human plasma and bile [14,15]. Future research will be needed to clarify the role for bile and vitamin D glucuronides in the 1,25(OH)2D-mediated activity of the intestinal epithelium.
In conclusion, we have confirmed that the intestinal 1,25(OH)2D-mediated response occurs in enterocytes at the villi of the duodenum and the tips of the colon by in situ observation of 1,25(OH)2D-stimulated mRNA. These data correspond with previous work using protein immunohistochemistry. Lastly, we noted a lack of stimulation in the duodenum despite increased plasma [1,25(OH)2D] and considered the possibility that the location of the response may indicate a physiological mechanism of enterohepatic signaling for 1,25(OH)2.
Highlights.
RNAScope ISH of 1,25(OH)2D-stimulated mouse intestines.
1,25(OH)2D induces expression of Cyp24a1 mRNA in only epithelial cells of the duodenum and colon.
The 1,25(OH)2D-mediated response occurs in villi or tips of the intestine rather than crypt regions.
An increase in plasma [1,25(OH)2D] did not affect the response in the duodenum.
Acknowledgements
The authors would like to thank Catherine Martens for invaluable technical assistance in performing these studies. This work was supported in part by the National Institutes of Health [CA 173628].
Abbreviations:
- 1,25(OH)2D
1,25-dihydroxyvitamin D3
- β-G-1,25(OH)2D
β-glucuronic-1,25-dihydroxyvitamin D3
- Cyp24a1
cytochrome P450 family 24 subfamily A member 1
- VDR
vitamin d receptor
- VDRE
vitamin D response element
- ISH
in situ hybridization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interests
The β-G-1,25(OH)2D used in this study was supplied by Glycomyr (Ames, IA), a company jointly owned by authors JPG and RLH. Remaining authors, CJR, NJK, and DCB, do not have conflicts to disclose.
References
- [1].Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G, Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects, Physiol. Rev 96 (2015) 365–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Norman AW, The history of the discovery of vitamin D and its daughter steroid hormone, Ann. Nutr. Metabol 61 (2012) 199–206. [DOI] [PubMed] [Google Scholar]
- [3].Goff JP, Koszewski NJ, Haynes JS, Horst RL, Targeted delivery of vitamin D to the colon using beta-glucuronides of vitamin D: therapeutic effects in a murine model of inflammatory bowel disease, Am. J. Physiol. Gastrointest. Liver Physiol 302 (2012) G460–G469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Koszewski NJ, Horst RL RL, Goff JP, Importance of apical membrane delivery of 1,25-dihydroxyvitamin D3 to vitamin D-responsive gene expression in the colon, Am. J. Physiol. Gastrointest. Liver Physiol 303 (2012) G870–G878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lee SM, Riley EM, Meyer MB, Benkusky NA, Plum L, DeLuca HF, Pike JW, 1,25-dihydroxyvitamin D3 controls a cohort of vitamin D receptor target genes in the proximal intestine that is enriched for calcium-regulating components, J. Biol. Chem 290 (2015) 18199–18215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jones G, Prosser DE, Kaufmann M, 25 - Hydroxyvitamin D-24-hydroxylase (Cyp24a1): Its important role in the degradation of vitamin D, Arch. Biochem. Biophys 525 (2012) 9–18. [DOI] [PubMed] [Google Scholar]
- [7].Zierold C, Darwish HM, DeLuca HF, Two Vitamin D Response Elements Function in the rat 1,25-Dihydroxyvitamin D 24-hydroxylase promoter, J. Biol. Chem 270 (1995) 1675–1678. [DOI] [PubMed] [Google Scholar]
- [8].Boos A, Riner K, Hassig M, Liesegang A, Immunohistochemical demonstration of vitamin D receptor distribution in goat intestines, Cells Tissues Organs 186 (2007) 121–128. [DOI] [PubMed] [Google Scholar]
- [9].Colston KW, Mackay AG, Finlayson C, Wu JCY, Maxwell JD, Localisation of vitamin D receptor in normal human duodenum and in patients with coeliac disease, Gut 35 (1994) 1219–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sidler-Lauff K, Boos A, Kraenzlin M, Liesegang A, Influence of different calcium supplies and a single vitamin injection on vitamin D receptor and calbindin D9k immunoreactives in the gastrointestinal tract of goat kids, J. Anim. Sci 88 (2010) 3598–3610. [DOI] [PubMed] [Google Scholar]
- [11].Wang Y, Zhu J, DeLuca HF, Where is the vitamin D receptor?, Arch. Biochem. Biophys 523 (2012) 123–133. [DOI] [PubMed] [Google Scholar]
- [12].Baker M, RNA imaging in situ, Nat. Methods 9(8) (2012) 787–790. [Google Scholar]
- [13].Fleet JC, Schoch RD, Molecular mechanisms for regulation of intestinal calcium absorption by vitamin D and other factors, Crit. Rev. Clin. Lab. Sci 47:4 (2010) 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gao C, Bergagnini-Kolev MC, Liao MZ, Wang Z, Wong T, Calamia JC, Lin YS, Mao Q, Thummel KE, Simultaneous quantification of 25-hydroxyvitamin D3-3-sulfate and 25-hydroxyvitamin D3-3- glucuronide in human serum and plasma using liquid chromatography-tandem mass spectrometry coupled with DAPTAD-derivatization, J. Chromatogr. B 1060 (2017) 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang Z, Wong T, Hashizume T, Dickmann LZ, Scian M, Koszewski NJ, Goff JP, Horst RL, Chaudhry AS, Schuetz EG, Thummel KE, Human UGT1A4 and UGT1A3 conjugate 25-hydroxyvitamin D3: Metabolite structure, kinetics, inducibility, and interindividual variability, Endocrinol 155:6 (2014) 2052–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]


